Drug survival and the associated predictors in South Korean patients with rheumatoid arthritis receiving tacrolimus
Article information
Abstract
Background/Aims
To investigate the drug survival rate of tacrolimus (TAC) and analyze the potential predictors of this rate in patients with rheumatoid arthritis (RA) in routine care.
Methods2018-01-16
In this retrospective longitudinal study, we enrolled 102 RA patients treated with TAC from April 2009 to January 2014 at a tertiary center in South Korea. The causes of TAC discontinuation were classified as lack of efficacy (LOE), adverse events (AEs), and others. The drug survival rate was estimated using the Kaplan-Meier method and the predictors of this rate were identified by Cox-regression analyses.
Results
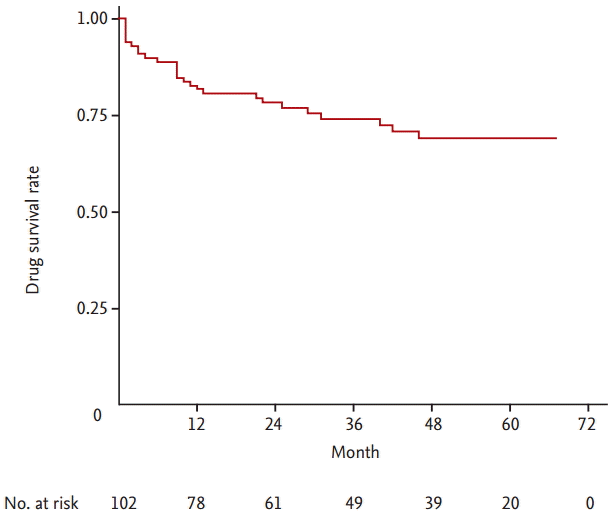
TAC was discontinued in 27 of 102 RA patients (26.5%). The overall 1-, 2-, 3-, and 4-year TAC continuation rates were 81.8%, 78.4%, 74.2%, and 69.1%, respectively and the median follow-up period from the start of TAC was 32.5 months. The number of TAC discontinuations due to LOE, AEs, and others were 15 (55.6%), 11 (40.7 %), and 1 (3.7%), respectively. The baseline high disease activity was a significant risk factor for TAC discontinuation after adjusting for confounding factors (hazard ratio [HR], 2.49; 95% confidence interval [CI], 1.16 to 5.35; p = 0.019). In addition, underlying interstitial lung disease was significantly associated with TAC withdrawal due to AEs (HR, 3.49; 95% CI, 1.06 to 11.46; p = 0.039).
Conclusions
In our study, TAC showed a good overall survival rate in patients with RA in real clinical practice. This suggests that the long-term TAC therapy has a favorable efficacy and safety profile for treating RA.
INTRODUCTION
Drug survival or retention has been reported as a composite measure of efficacy, safety, and tolerability in clinical practice [1,2]. Because of its chronicity and incurability, rheumatoid arthritis (RA) usually requires life-long therapy with disease modifying anti-rheumatic drugs (DMARDs); thus, long term retention of a DMARD is an important indicator of the performance of that drug in terms of managing of RA [3]. In light with this notion, growing attention has been paid to survival rate of various drugs in patients with RA from numerous national-wide registries or observational data reflecting daily practice. Among them, tumor necrosis factor α (TNF-α) inhibitors have been most extensively investigated [1-15], but relevant studies regarding tocilizumab and abatacept also have also been published in recent years [9,13,16]. Although more patients with RA were treated with conventional synthetic DMARDs than with biological agents in real practice, relatively little attention has been paid recently to the drug survival rate of these drugs, except for leflunomide [17-20].
Tacrolimus (TAC, FK506), a macrolide derived from Streptomyces tsukubaensis, was initially developed and used in organ transplantation [21]. It exerts an immunosuppressive action by inhibiting T-cell proliferation. After binding to the FK-binding protein, TAC inhibits calcineurin phosphatase and subsequently prevents translocation of the nuclear factor of activated T-cells which is needed for the production of cytokines such as interleukin 2 and interferon γ [21]. Because T-cell activation has a key role in the pathogenesis of RA, randomized clinical trials for TAC monotherapy or in combination therapy with methotrexate (MTX) in patients with RA have shown the remarkable efficacy and safety [22-25]. Thus, TAC can be applied as an additional therapeutic option for RA [21,26]. After TAC was approved for the first time in the world for treating RA in Canada in February 2004, it was also available as a DMARD for patients with RA in Japan and South Korea in April 2005 and October 2008, respectively. Then, several observational studies regarding the tolerability and effectiveness including radiographic changes of TAC treatment in Japanese patients with RA in various clinical settings, have been published [27-30]. However, long-term follow-up data about drug survival and the associated factors among patients with RA receiving TAC are still lacking to date, especially in South Korea. Therefore, the present study aimed to investigate the drug survival rate of TAC in the treatment of RA and to analyze the potential predictors of this rate in routine clinical care.
METHODS
Study design and subjects
We conducted a retrospective longitudinal study among consecutive 102 patients with RA who were treated with TAC (Prograf, Astellas Pharma, Tokyo, Japan) and followed at least 1 year follow-up from April 2009 to January 2014 at a university-affiliated rheumatology center in South Korea. All study subjects fulfilled the 1987 American College of Rheumatology classification criteria for RA [31] and they were followed longitudinally through reviewing of their entire medical records (inpatient and outpatient) until January 2015. The procedures of our study were approved by the Research and Ethical Review Board of the Pusan National University Hospital, which waived the need for informed patient consent (approval no.: E-2015076).
For the study subjects, the baseline clinical and laboratory parameters including their age at diagnosis, disease duration, sex, rheumatoid factor (RF) and anti-cyclic citrullinated peptide (anti-CCP) antibody status, number of previous DMARDs, concomitant medication (i.e., DMARDs, glucocorticoids, and nonsteroidal anti-inflammatory drugs), comorbid interstitial lung disease (ILD) status, swollen joint count (SJC), tender joint count (TJC), patient’s general health visual analog scale score (rated from 0 to 100), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) level were obtained and recorded. TAC treatment data such as the initial dosage and date of initiation and/or termination were also reviewed. Disease duration was defined as the difference between the date of diagnosis and the date of TAC initiation. ILD was defined as bibasilar fibrosis on chest radiography and/or fibrosis or ground-glass opacity on high resolution chest computed tomography in the absence of other causes. RF was assessed by a particle-enhanced immunoturbidimetric assay (range, 0 to 14 IU/mL) and anti-CCP was measured using a chemiluminescent microparticle immunoassay (range, 0 to 5 U/mL). The CRP level was measured by a particle-enhanced immunoturbidimetric assay (Tina-quant CRP, Roche Diagnostics, Basel, Switzerland) using a P-800 Modular analyzer (Roche Diagnostics). The Disease Activity Score assessed by using 28-joint counts for swelling and tenderness (DAS28)-ESR score was calculated by the following formula: DAS28-ESR score = 0.56 × √TJC 28 + 0.28 × √SJC 28 + 0.70 × lnESR+ 0.0014 × visual analog scale score [32].
A DAS28-ESR of > 5.1 defines high disease activity; 3.2 < DAS28-ESR ≤ 5.1 is a moderate disease activity; 2.6 < DAS28-ESR ≤ 3.2 is a low disease activity; DAS28-ESR ≤ 2.6 is a remission [33].
The main outcome of the present study was TAC discontinuation of any cause. The causes of TAC discontinuation were classified as follows: (1) lack of efficacy (LOE), (2) adverse events (AEs), and (3) others (e.g., patient or medical decision and miscellaneous reasons), which were judged based on the patient’s medical records. The time to TAC discontinuation was calculated from the date of TAC initiation to the first treatment interruption. Interruptions were considered to be definitive when no consecutive re-introduction of TAC was recorded based on medical chart reviews [15].
Statistical analysis
Data are summarized as the mean and standard deviation (with normal distribution) or median and interquartile range (with non-normal distribution) for continuous variables, and a number of cases with percentages for categorical variables, as appropriate. The Kolmogorov-Smirnov tests were used to assess the normal distribution of data. The drug survival rates of TAC were calculated and plotted using Kaplan-Meier curves and compared using the log-rank test. To investigate the potential predictors of TAC discontinuation, we used multivariable Cox-proportional hazard regression analyses with backward model selection of demographic variables such as age at TAC initiation and sex as well as variables that had a p ≤ 0.1 in univariable analysis. All statistical tests were two-sided and p values less than 0.05 were considered statistically significant. All analyses were performed using PASW version 18.0 (SPSS Inc., Chicago, IL, USA) and STATA version 11.0 (StataCorp LP, College Station, TX, USA).
RESULTS
Table 1 shows the baseline clinical characteristics of patients with RA treated with TAC. The mean age at the initiation of TAC was 54.2 ± 13.3 years and the median disease duration was 34 months (range, 8 to 73). Most patients were female (77.5%) with a positive RF or anti-CCP antibody and the frequency of ILD was 20.6%. The mean DAS28-ESR score at the initiation of TAC treatment was 4.83 ± 1.13 and the numbers of RA patients with high, moderate and low disease activity were 38 (37.3%), 55 (53.9%), and 8 (7.8%), respectively. Although five patients with RA were treated with TAC only, most study subjects were receiving TAC plus DMARDs combination therapy; the most common concomitant DMARDs was MTX. In addition, most patients with RA were treated with one or more DMARDs before TAC therapy, but TAC was started at the diagnosis of RA in seven subjects (6.9%). The mean initial and last dose of TAC during the study period were 1.76 ± 0.25 and 1.93 ± 0.39 mg, respectively.
Twenty-seven patients (26.5%) discontinued TAC treatment among 102 patients with RA. The overall 1-, 2-, 3-, and 4-year TAC continuation rates were 81.8%, 78.4%, 74.2%, and 69.1%, respectively and the highest discontinuation rate was within the first year after TAC initiation (Fig. 1). The median follow-up period from the start of TAC was 32.5 months (range, 12 to 55.3). As shown in Fig. 2, the drug survival rate of patients with a high disease activity was significantly lower than those with a low to moderate disease activity (p = 0.014). The most common cause of TAC discontinuation was LOE (55.6%), whereas AEs resulting in TAC discontinuation occurred in 11 patients (40.7%) with RA (Table 2). Four patients with RA stopped TAC because of gastrointestinal disorders including nausea and diarrhea. Cardiopulmonary disorders leading to TAC withdrawal such as ILD exacerbation and angina pectoris occurred in three patients. Preceding bronchitis, especially, was regarded to have triggered ILD exacerbation in two patients. Allergic reaction, peripheral neuropathy, and hyperkalemia developed in two, one, and one subjects, respectively. However, all AEs were mild or moderate and resolved after discontinuing TAC and subsequent appropriate management. In addition, TAC was not discontinued owing to renal impairment or glucose metabolism abnormality. One patient with RA stopped TAC 3 months after initiation because of the cost of the medicine.

Comparisons of the discontinuation rate of tacrolimus according to the disease activity of the patients.
Table 3 summarizes several significant predictors for TAC discontinuation in patients with RA using Cox-proportional regression models. The baseline high disease activity (hazard ratio [HR], 2.49; 95% confidence interval [CI], 1.16 to 5.35; p = 0.019) was the independent risk factor for TAC withdrawal due to any cause after adjusting for confounding factors. However, the initial or last dose of TAC, concomitant MTX treatment, number of previous DMARDs, disease duration and RF or anti-CCP antibody positivity were not related to drug survival of TAC. In addition, concomitant sulfasalazine or hydroxychloroquine therapy and the dose of MTX did not have a significant association with TAC discontinuation (data not shown). We also investigated predictors for cause-specific TAC discontinuation. The baseline high disease activity and age at the TAC initiation less than 60 years were significant predictors for TAC withdrawal due to LOE. Of interest, RA patients with ILD showed significantly lower TAC retention due to AEs after adjusting for confounding factors (HR, 3.49; 95% CI, 1.06 to 11.46; p = 0.039). However, age at the TAC initiation, initial dose of TAC and concomitant MTX use did not show the statistically significant association with the increased TAC discontinuation rate due to AEs. In addition, the number of previous DMARDs was not related to TAC survival due to any cause, LOE, and AEs (data not shown).
DISCUSSION
The present study investigated the long term retention rate of TAC used for patients with RA in clinical practice. Our study demonstrated that the drug survival rates of TAC after 1, 2, 3, and 4 year were 81.8%, 78.4%, 74.2%, and 69.1%, respectively with a median follow-up duration of 32.5 months in patients with RA. The highest rate of TAC discontinuation occurred in the first year. The baseline high disease activity, defined as a DAS28-ESR of > 5.1, was a significant predictor for TAC discontinuation. The two main reasons for TAC withdrawal were LOE and AEs, and more patients with RA discontinued TAC due to LOE (55.6%) than due to AEs (40.7%). The age at the TAC initiation less than 60 years and the baseline high disease activity were associated with TAC discontinuation due to LOE, whereas underlying ILD was a risk factor for worse survival of TAC due to AEs.
Drug survival is known to be a surrogate marker of the long-term efficacy and safety of a drug in real clinical practice, but patient’s and physician’s satisfaction with the treatment, cost, convenience, and availability of therapeutic alternatives can also influence the drug retention rate. Because RA requires lifelong treatment to control inflammation and joint damage, DMARDs with a high retention may be an optimal choice in managing RA. In previous studies, the 1 and 2 year survival rate of anti-TNF-α agents in patients with RA approximately ranged from approximately 60% to 80% and 40% to 70%, respectively [1-15]. When compared with TNF-α inhibitors, non-TNF-α biologic agents such as tocilizumab and abatacept showed similar rates of retention in the observational studies [9,13,16]. With respect to conventional synthetic DMARDs, the 2-year continuation rate of leflunomide varies from 44.7% to 71%, but two recent studies conducted in the early 2010s demonstrated a better retention rate with leflunomide than that found in previous data [18-20]. Our finding of a 2-year TAC survival of 78.4% in RA patients seems to be comparable to that of leflunomide or even biologic agents. Although comparing the survival rate between TAC and other DMARDs should be performed cautiously because of the difference in the baseline clinical features, including the disease activity between the previous studies and ours, our data suggests that TAC has a good long term effectiveness and tolerability of TAC in the treatment of RA in real clinical practice. However, further investigations are needed to compare the persistence rate between TAC and other agents.
To our knowledge, only one previous study has calculated the survival figures for TAC in patients with RA. Ogasawara et al. [28] reported that the 1 and 2 year survival rates of TAC in 115 Japanese patients with RA were 57.9% and 48.9%, respectively, which were lower than those in our study. The baseline disease activity, considered as a risk factor for TAC discontinuation in our study, was comparable between the Ogasawara et al. [28]’s study and ours. Thus, the discrepancy of TAC survival between both studies may be related to the difference in ethnicity among the study subjects, practice guidelines or the incidence of AEs leading to TAC withdrawal. Compared with our data, Ogasawara et al. [28]’s study had a shorter follow-up period and did not evaluate the cause specific survival of TAC. Hence, our study provided more comprehensive data on TAC survival in clinical practice. Kitahama et al. [27] and Kanzaki et al. [29] also assessed the retention of TAC in Japanese patients with RA. However, these studies did not show the exact figures for the survival rate and cannot be generalized because of short follow-up period (12 months) and the small sample size (n = 24) in Kitahama et al. [27]’s and Kanzaki et al. [29]’s studies, respectively.
In our study, the most common cause of TAC discontinuation was LOE (55.6%), followed by AEs (40.7%). Similarly, previous observational data showed that LOE was the more common cause of discontinuation of anti-TNF-α agents than AEs in patients with RA [3,4,14], although some studies have reported contradictory results [8,12]. Gomez-Reino et al. [7] demonstrated that patients with RA starting TNF-α inhibitors more recently had a higher risk of discontinuation due to LOE, compared with earlier studies, whereas the survival rate due to AEs had remained stable. Because of the adaptation of more aggressive treatment strategies, development of newer efficacious biologic agents and higher expectation about the response to therapy or remission, switching between biologic agents occurred more frequently in recent years according to previous data [7,34]. This idea was consistent with our finding that LOE was the main cause of TAC discontinuation, considering that most of our RA patients had an inadequate response to one or more DMARDs before TAC therapy.
Since our study included more than 100 patients with RA long follow-up period, investigating the predictors for TAC survival was possible. The baseline high disease activity was an independent risk factor for TAC discontinuation in the present study. Furthermore, in the cause-specific analyses, a high disease activity was associated with TAC withdrawal due to LOE, but not AEs. We can infer from this finding that RA patients with a high disease activity would have had more of a chance to discontinue TAC, especially due to an inadequate treatment response. The relationship between a higher DAS28 and worse survival of anti-TNF-α agents was also observed in the previous studies [4,7]. In addition, previous data showed a dose-dependent response to TAC in patients with RA [22], but the initial or last dose of TAC was not significantly associated with drug survival in our study. As the initial or last dose of TAC usually ranged from 1.5 to 2.5 mg, the dose-dependent manner of TAC survival was not shown in our data. Of interest, age at the TAC initiation less than 60 years was a significant predictor for worse TAC survival due to LOE in our study, which was contrary to the results of previous studies regarding the survival of anti-TNF-α agents and leflunomide [7,10,19]. The inverse relationship between the baseline age and TAC discontinuation in our study may be explained by the higher expectation for the treatment outcome in younger patients with RA or the difference of TAC efficacy according to age. However, because of the lack of data concerning the association between the efficacy of TAC and the baseline age, this finding remains controversial and needs to be further confirmed by additional studies. In addition, concomitant MTX treatment was reported to increase the retention rate of anti-TNF-α agents and leflunomide [2,6,19], but these trends were not found in the present study.
Another significant finding in our study was that underlying ILD was related to TAC withdrawal due to AEs. Some of the most frequently prescribed DMARDs such as MTX, leflunomide, and anti-TNF-α agents have been implicated with pulmonary toxicities including ILD exacerbation [35]. Otherwise, calcineurin antagonists can be used as a therapeutic option to treat ILD associated with connective tissue disorders including idiopathic inflammatory myopathies [35,36]. Thus, it has been presumed that TAC may be safer in patients with RA-ILD than other DMARDs such as MTX. However, recent case reports raised a possibility of TAC induced pulmonary injury in patients with RA, particularly with pre-existing ILD [37]. Additionally, in the post-marketing surveillance of TAC in 3,267 Japanese patients with RA, ILD was reported to occur in 17 cases (0.5%) [38]. Taken together with our finding, careful attention needed to be paid to RA patients with ILD who are receiving TAC.
We note a number of potential limitations to our study. First, we retrieved the drug survival data for TAC only; thus, direct comparisons between TAC and other DMARDs were not possible. However, a majority of previous studies on drug survival have analyzed a single agent, except for studies on TNF-α inhibitors [16-19,27,29]. Thus, we think that our data also provide useful clinical information regarding the long-term retention rate of TAC. As mentioned above, further research is needed to compare the persistence rate between TAC and other DMARDs. Second, unmeasured confounding factors such as the clinician’s personal preference and economic burden of TAC cannot be completely excluded. In South Korea, TAC is the most expensive conventional DMARD and it costs approximately $3.30 per 1 mg capsule. In general, patients have a tendency to have higher expectations for more expensive drugs, which may have affected our results. Third, because of the retrospective study design, some information such as the follow-up disease activity after TAC therapy could not be retrieved. Fourth, because of the small number of subjects who were treated with TAC monotherapy, it was impossible to compare the drug survival rate between TAC monotherapy and combination treatment with other DMARDs. This may have also contributed to the lack of statistical association between concomitant MTX treatment and better TAC survival in our study. Lastly, we recruited all of our patients with RA from a single tertiary hospital, which could have resulted in a selection bias.
In summary, TAC showed a good overall survival rate in patients with RA in real clinical practice. This suggests that the long-term TAC therapy has a favorable efficacy and safety profile for treating RA. The baseline high disease activity was a risk factor for TAC discontinuation, similarly with anti-TNF-α agents. In addition, underlying ILD was significantly associated with TAC withdrawal due to AEs. Therefore, careful evaluation of pulmonary toxicities may be needed in patients with RA receiving TAC. Although TAC has not been widely used as DMARDs in patients with RA in many Western countries, TAC may be a good option for managing RA in terms of long-term drug survival based on our data.
KEY MESSAGE
1. Tacrolimus (TAC) showed a good overall survival rate in patients with rheumatoid arthritis (RA) in real clinical practice, suggesting the favorable long-term efficacy and tolerability of TAC for treating RA.
2.The baseline high disease activity was a significant risk factor for TAC discontinuation.
3. Underlying interstitial lung disease was significantly associated with TAC withdrawal due to adverse events.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by clinical research grant from Pusan National University Hospital 2015. We specially thank the late Professor Sung-Il Kim who devoted himself to education, research, and patient care in Division of Rheumatology, Department of Internal Medicine, Pusan National University School of Medicine (1963 to 2011).