Mean platelet volume: a potential biomarker of the risk and prognosis of heart disease
Article information
Abstract
Platelets are essential for progression of atherosclerotic lesions, plaque destabilization, and thrombosis. They secrete and express many substances that are crucial mediators of coagulation, inflammation, and atherosclerosis. Mean platelet volume (MPV) is a precise measure of platelet size, and is routinely reported during complete blood count analysis. Emerging evidence supports the use of MPV as a biomarker predicting the risk of ischemic stroke in patients with atrial fibrillation, and as a guide for prescription of anticoagulation and rhythm-control therapy. In addition, MPV may predict the clinical outcome of percutaneous coronary intervention (PCI) in patients with coronary artery disease and indicate whether additional adjunctive therapy is needed to improve clinical outcomes. This review focuses on the current evidence that MPV may be a biomarker of the risk and prognosis of common heart diseases, particularly atrial fibrillation and coronary artery disease treated via PCI.
INTRODUCTION
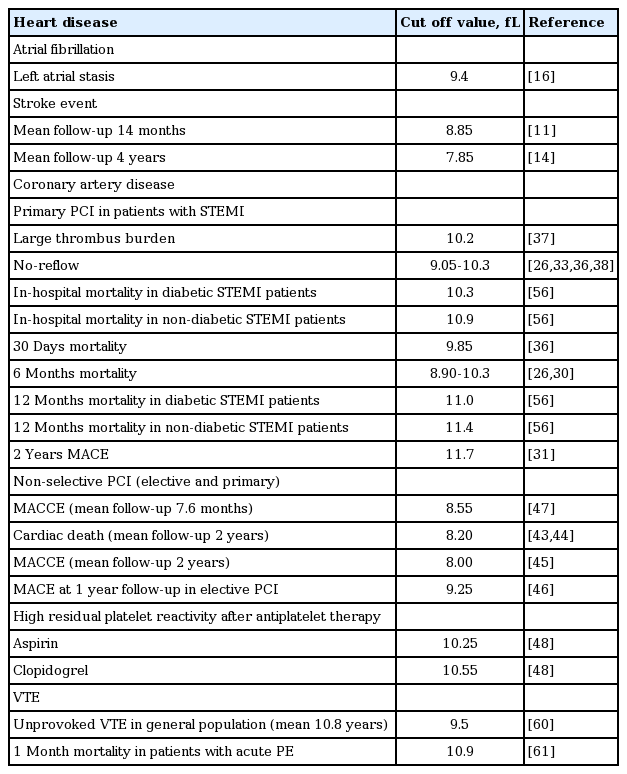
Platelets are small, anucleate cytoplasmic cells that lack genomic DNA and have a volume of about 7 to 11 fL. They contain many organelles, a microtubular system, a metabolically active membrane, and have two types of granules. The α granules contain the von Willebrand factor, platelet-derived growth factor, platelet factor 4, and β-thrombomodulin; the dense platelet bodies contain adenosine nucleotides (adenosine diphosphate [ADP] and adenosine triphosphate), calcium, and serotonin. Platelets play essential roles in the progression of atherosclerotic lesions, plaque destabilization, and thrombosis; they express and secrete many substances that are crucial mediators of coagulation, inflammation, and atherosclerosis [1,2]. Larger platelets are enzymatically and metabolically more dynamic than smaller ones, and they exhibit greater prothrombotic potential [3,4]. Increased platelet size is linked to other markers of activity, including platelet aggregation, enhanced thromboxane synthesis and β-thromboglobulin release, and increased expression of adhesion molecules [5]. Consequently, platelet volume is thought to be predictive of cardiovascular disease [6]. Therefore, the mean platelet volume (MPV) has been explored as a possible indicator of platelet reactivity and a predictor of various diseases [7], particularly cardiovascular disease (Table 1). MPV is a precise measure of platelet size, being measured via electrical impedance using automated hematological analyzers. MPV is a routine component of a complete blood count. This review explores the evidence that MPV is a biomarker of the risk and prognosis of common heart diseases, particularly atrial fibrillation (AF) and diseases treated via percutaneous coronary intervention (PCI).
EVIDENCE THAT THE MPV IS A BIOMARKER OF ISCHEMIC STROKE RISK IN AF PATIENTS
MPV and prediction of stroke risk
AF is the most common major cardiac arrhythmia, and it is associated with significant morbidity and mortality from ischemic stroke [8,9]. There is a positive association between MPV and the severity of acute ischemic stroke [10], and MPV is useful for predicting the risk of ischemic stroke in patients with AF [11-15]. A recent case-control study showed that stroke patients with AF have a higher MPV than AF patients without a stroke history [13]. The precise mechanism underlying the supposed relationship (cardiac embolism caused by increased platelet reactivity) remains to be fully elucidated. However, Providencia et al. [16] recently suggested an association between MPV and markers of left atrial stasis, reinforcing the notion that a cardioembolic mechanism may be in play when AF is associated with stroke. In that study, MPV independently predicted the development of a left-atrial appendage thrombus. The MPV cutoff values for predicting ischemic stroke in AF patients were 7.85 to 8.85 fL (Table 1).
Use of the MPV to guide anticoagulant therapy in AF patients
Anticoagulant therapy reduces the risk of stroke by 40% in patients with AF, and is more effective than antiplatelet therapy [17]. Hence, identifying patients at higher risk for ischemic stroke is essential to optimize treatment. The MPV has been shown to enhance the predictive value of the clinical variables employed when calculating CHADS2 or CHA2DS2 VASc scores [11,12]. One study found that the cutoff MPV better predicted ischemic stroke in those with a lower CHADS2 score (< 2) than in those with a higher score (≥ 2) [11]. In addition, those with a high MPV who were not on anticoagulation therapy experienced poorer stroke-free survival than did others; this was true even for patients with CHADS2 scores < 2. It was suggested that anticoagulation therapy was required by patients with high MPVs, even those in the low to intermediate risk groups. Similarly, another study suggested that measurement of MPV may help physicians to decide whether to administer anticoagulants to patients with CHA2DS2 VASc scores of 1, who are thus at intermediate risk of stroke [12].
MPV as a potential guide toward rhythm or rate control strategies in AF patients
Treatment of AF with rhythm or rate control therapy is tailored to patient inclinations and characteristics [18]. Many randomized controlled clinical trials have compared rhythm and rate control strategies in patients with AF, and have found no differences in mortality [19-23]. Nevertheless, the appropriate choice of patients who need rate or rhythm control treatment (to prevent ischemic stroke) among those with AF remains a matter of contention [24,25]. To the best of our knowledge, only one study, which was both retrospective in nature and had a small sample size, has explored whether MPV could potentially guide the provision of rhythm or rate control therapy to AF patients [14]. In this work, the rate control strategy used to treat AF, and a high MPV, were independent predictors of ischemic stroke, as was a high CHADS2 score (≥ 2). Such an association was also evident in patients at high risk for ischemic stroke as indicated by their MPVs and CHADS2 scores. This suggests that implementation of a rhythm control strategy might be necessary in patients with a high MPV or CHADS2 score (≥ 2).
EVIDENCE THAT MPV IS A BIOMARKER OF THE RISK AND PROGNOSIS OF CORONARY ARTERY DISEASES
Clinical assessment of MPV is ongoing. Currently, three lines of evidence suggest that it is potentially a clinically useful biomarker for risk stratification of patients who may develop coronary artery disease after PCI (Table 1). First, several studies have addressed the frequencies of impaired reperfusion, left ventricular systolic dysfunction, and mortality in patients with acute myocardial infarction (AMI) who have undergone primary PCI or thrombolysis [26-39]. Second, some studies have shown that MPV is a useful predictive marker of short- and long-term clinical outcomes in unselected PCI cohorts, regardless of whether the patients had undergone elective or primary PCI [40-47]. Third, some reports have suggested that MPV, or changes in it over time, reflect high residual platelet reactivity after conventional dual antiplatelet therapy in patients who have undergone PCI [47-49].
MPV and AMI
MPV increases during AMI and in the weeks thereafter [50-52]. MPV is associated with markers of platelet activity, particularly the expression levels of the glycoprotein Ib and glycoprotein IIb/IIIa receptors [53,54]. An elevated MPV correlates with poor clinical outcomes among survivors of MI in the era of thrombolysis, and an impaired response to thrombolysis in those with ST segment elevation myocardial infarction (STEMI) [28,55]. MPV is also a strong independent predictor of impaired angiographic reperfusion, in-hospital major adverse cardiovascular events, and 30-day, 6-month, 12-month, and 2-year mortality from STEMI treated via primary PCI [26,30-33,36-38,56]. In addition, a higher MPV on admission is independently associated with impaired microvascular perfusion, a poor postintervention myocardial blush grade, decreased post-PCI thrombolysis, and a poorer myocardial infarction flow grade (thrombolysis in myocardial infarction [TIMI]) in STEMI patients treated via primary PCI [33-36,57]. In one study, a higher MPV on admission was strongly associated with greater microvascular resistance, a steeper diastolic deceleration time, a lower thermodilution-derived coronary flow reserve, and a higher coronary wedge pressure [57]. MPV seems to play a role in mediating reperfusion injury. In patients with STEMI scheduled for PCI, MPV at admission may be a valuable discriminator of a higher-risk patient subgroup, and a useful guide when deciding whether adjunctive therapy may be necessary to improve outcomes [27]. MPV cutoff values for predicting poor clinical outcomes in STEMI patients treated via PCI are 8.9 to 11.7 fL; thus, somewhat higher than those predictive of ischemic stroke in AF patients (Table 1).
MPV and clinical outcomes after PCI
Duygu et al. [40] suggested a role for MPV as a useful hematological marker allowing early and simple identification of patients with stable coronary artery disease at high risk for post-PCI low-reflow. MPV independently predicted the post-PCI-corrected TIMI frame count. Another study found that MPV predicted in-stent restenosis in patients undergoing PCI [58]. Similar to the effects of MPV on AMI, several studies have found that an elevated MPV is a strong independent predictor of long-term outcomes after PCI [41-47]. However, one study found that mortality increased when the MPV rose over time after PCI, but the pre-procedural MPV was not predictive in this context [59]. It was suggested that monitoring of MPV after PCI might aid risk stratification. MPV cutoffs for predicting poor clinical outcomes in patients with unselected coronary artery disease treated via PCI are 8.00 to 9.25 fL (Table 1) [11,14,16,26,30,31,33,36-38,43-48,56,60,61].
MPV and residual platelet reactivity in patients on dual antiplatelet therapy
Antiplatelet therapy reduces the incidence of both procedure-related complications and ischemic cardiovascular events after PCI [62,63]. Particularly, dual antiplatelet therapy (aspirin and an ADP receptor inhibitor) is the present standard of care after implantation of drug-eluting stents. Nevertheless, high residual platelet reactivity can limit the utility of antiplatelet therapy, increasing the frequency of cardiovascular events both during the procedure and during long-term follow-up [64-67]. Recently, however, Paulu et al. [68], in a prospective observational study, showed that clopidogrel resistance was not of prognostic utility in an unselected cohort of 378 patients who underwent PCI. In addition, Collet et al. [69] observed no significant improvement in clinical outcomes when platelet function was monitored and adjusted in patients who underwent coronary stenting, compared to those who received standard antiplatelet therapy (without monitoring); this was a large, randomized open-label study on 2,440 patients. Platelet activity testing can be time-consuming, expensive, and technically complex [70]. However, MPV can be readily measured before PCI using automated hematology analyzers. Recently, Kim et al. [48] suggested that a high MPV was associated with reduced responses to aspirin and clopidogrel. Some investigators have suggested that an increase in MPV over time after PCI is associated with high on-treatment platelet reactivity [49]. Moreover, Choi et al. [47] suggested that MPV was superior to platelet function testing in terms of predicting cardiac death or cardiovascular events in patients who had undergone PCI, particularly those in an acute coronary syndrome subgroup.
COMPARISON OF THE PREDICTIVE UTILITY OF MPV AND OTHER TRADITIONAL BIOMARKERS IN TERMS OF CLINICAL OUTCOMES
Traditional biomarkers, including pulse wave velocity and the levels of high-sensitivity cardiac troponin T, N-terminal pro-B type natriuretic peptide, and C-reactive protein, have long been of clinical research interest because of the roles that they play in predicting the clinical outcomes of patients with heart disease. Recently, some studies have suggested that a high MPV has a predictive utility comparable to those of other biomarkers (Table 2, Fig. 1) [43-45].
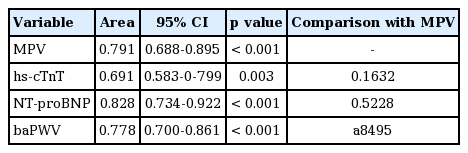
MPV, hs-cTnT, NT-proBNP level, and baPWV to predict cardiac death after percutaneous coronary intervention
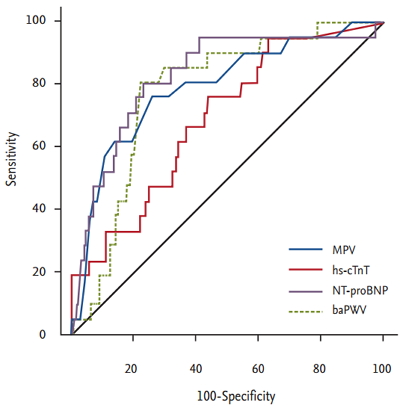
Receiver operating characteristic curve for mean platelet volume (MPV), high-sensitivity cardiac troponin T level (hs-cTnT), N-terminal pro-B type natriuretic peptide (NT-proBNP) level, and brachial-ankle pulse wave velocity (baPWV) to predict cardiac death after percutaneous coronary intervention. Adapted from Ki et al. [43], with permission from Taylor & Francis and Seo et al. [44], with permission from Taylor & Francis.
LIMITATIONS OF MPV
MPV can be simply and inexpensively determined and does not require professional interpretation. However, there are several limitations to using MPV as an indicator of heart disease. This is because most relevant studies have been retrospective in nature, enrolled small numbers of patients, or had confounding factors that may have affected platelet volume [71]. Furthermore, a wide range of cut-off values has been used in retrospective studies, emphasizing that prospective works are needed. MPV increases as blood is stored in ethylenediaminetetraacetic acid, and the reliability of MPV measurement gradually decreases after 4 hours of such storage [72,73]. Therefore, MPV should be measured within 4 hours of sampling to exclude the possibility of storage-related errors. Notwithstanding the potential clinical utility of MPV, these issues, together with methodological problems in MPV assessment, continue to be important limitations.
CONCLUSIONS
Many studies have shown associations between an elevated MPV, the risk of ischemic stroke in AF patients, and poor clinical outcomes after PCI in patients with coronary artery disease. This marker can help physicians to identify patients at high risk for ischemic stroke, who thus require anticoagulation therapy, and AF patients who need rhythm control therapy. The marker also affords valuable insights into how to identify patients at high risk for coronary artery disease after PCI, and provides useful guidance as to when additional adjunctive therapy is needed to improve clinical outcomes. Much remains to be learned about MPV. It is essential to explore whether therapeutic adjustment of the marker improves cardiovascular care.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning (2016R1A2B4011905) and the Ministry of Education, Science and Technology (2014028083).