Identification of distinctive clinical significance in hospitalized patients with endoscopic duodenal mucosal lesions
Article information
Abstract
Background/Aims
Duodenitis is not infrequent finding in patient undergoing endoscopy. However, hospitalized patients have a higher incidence of secondary duodenal mucosal lesions that might be related with inflammatory bowel disease (IBD), cytomegalovirus (CMV) infection, tuberculosis, immunologic disorders, or other rare infections. We aimed to identify clinicopathologic features of duodenal mucosal lesions in hospitalized patients.
Methods
All hospitalized patients having duodenal mucosal lesions were identified by endoscopic registration data and pathologic data query from 2011 to 2014. The diagnostic index was designed to be sensitive; however, a detailed review of medical record and endoscopic findings was undertaken to improve specificity. Secondary duodenal lesion was defined as having specific reason to explain the duodenal lesion.
Results
Among 6,334 hospitalized patients have undergone upper endoscopy, endoscopic duodenal mucosal lesions was detected in 475 patients. Secondary duodenal lesions was 21 patients (4.4%) and the most frequent secondary cause was IBD (n = 7). The mean age of secondary group was significantly lower than that in primary group (42.3 ± 18.9 years vs. 58.5 ± 16.8 years, p = 0.00), and nonsteroidal anti-inflammatory drugs were less frequently used in secondary group, but there was no differences of gender or presence of Helicobacter pylori. The involvement of distal part of duodenum including postbulbitis or panduodenitis was more frequently detected in secondary group than in primary group. By multivariate regression analysis, younger age of 29 years and the disease extent were significant predictors for the secondary mucosal lesions.
Conclusions
Secondary duodenal mucosal lesions with different pathophysiology, such as IBD or CMV infection, are rare. Disease extent and age seems the most distinctive feature of secondary duodenal mucosal lesions.
INTRODUCTION
Duodenitis is defined as inflammation on the duodenal mucosa and is not infrequent finding in patient undergoing endoscopy [1-4]. Endoscopic examination with histopathological evaluation can provide the understanding of clinical meaningful lesions. Among 3,872 subjects who had undergone endoscopy for medical check-up, endoscopic duodenal mucosal lesions were detected in 21% and active/healing stage of duodenal ulcers were detected in 2.9% [5]. With expanded enforcement of endoscopy, many cases of inflammation on the duodenal mucosa are found among patients undergone endoscopy; however, its clinical significance is not definite.
In conventional concept, duodenitis has been perceived as precursor of duodenal ulcer and gastric hyperacidity is one of most significant causes in the spectrum of peptic ulcer disease [6,7]. Subsequently, Helicobacter pylori infection might have a strong causality in patients with duodenal ulcer; therefore, it has been suggested that gastric metaplasia in the duodenum and H. pylori associated gastritis may be synergistic in the pathogenesis of duodenitis [8]. However, the prevalence of endoscopic duodenitis was similar for each study as 9% to 16%, even though the prevalence of H. pylori infection varies from studies in 28.9% to 75% [8-11].
A variety conditions are known to cause duodenal mucosal lesions including inflammatory bowel disease (IBD), tuberculosis, immunologic disorders, or other rare infections [12-17], especially, these secondary causes of duodenal lesions might be frequently detected in hospitalized patients. Upper gastrointestinal (GI) endoscopy is a useful and relatively non-invasive technique to diagnose duodenal mucosal lesion by causes other than H. pylori infection. It allows direct visualization of the mucosa for the acquisition of targeted biopsies to evaluate the severity and extent of intestinal inflammation.
We aimed to identify clinical and endoscopic features in hospitalized patients with duodenal mucosal lesions and to evaluate the predictors of clinically significant secondary duodenal mucosal lesions.
METHODS
Case ascertainment
From October 2011 to October 2014, 6,334 patients underwent the upper GI endoscope among hospitalized person at Ewha Womans University Mokdong Hospital. Patients with duodenal mucosal lesions were identified using a medical records linkage system including endoscopic registration data and pathologic data query. A detailed chart review was undertaken to improve specificity by one experienced doctor (Y.H.) and ensure these cases actually had duodenal mucosal lesions.
We excluded patients younger than 18 years, patients with Brunner’s gland hyperplasia, duodenal polyps, cysts, or subepithelial lesions. Ethics approval was obtained from the Institutional Review Board of Ewha Womans University Mokdong Hospital (EUMC 2017-07-058). This study was conducted in retrospective medical record review; therefore, there was no need to obtain the patient consent.
Data collection and case definitions
We performed several steps to ensure reliability and high quality. All information obtaining to diagnosis was recorded in specially designed standardized clinical data form. Demographic data as well as clinical data were collected from the medical records. In any uncertain cases, the endoscopic or pathologic findings were discussed with an experienced endoscopist (H.K.J.) and a pathologist (S.P.). The following information was collected from the review of medical records: (1) demographics including age at time of diagnosis and gender; (2) GI symptoms, including epigastric pain, abdominal pain, nausea, vomiting, dizziness, indigestion, bloating, poor oral intake, and alarm symptoms such as hematemesis, melena, hematochezia, chronic vomiting and weight loss; (3) comorbidities, including hypertensions, diabetes mellitus, pulmonary tuberculosis, IBD, malignancy, autoimmune disease, chronic renal failure; and (4) any medications that might possibly induce duodenal mucosal lesions, such as nonsteroidal anti-inflammatory drugs (NSAIDs), aspirin, anti-platelet agents, steroid, immunosuppressant, proton pump inhibitors were recorded.
Assessment of endoscopic findings was recorded in terms of erythema, hemorrhage, erosion, edema, and ulcer according to the following definitions [18-20]: (1) erythema was graded as followings: 0, no lesion; 1, distinctive color change of the patch; 2, a little more clear change in color of the patch; and 3, beef like reddish color detected; (2) hemorrhage was graded by number of hemorrhagic spots: 0 to 1, 2 to 5, 6 to 10, and ≥ 10; (3) erosion was graded by number of lesions: no erosion, 1 to 2, 3 to 5, and ≥ 6; and (4) ulcer was categorized into three stage with its number: active, healing, and scar stage.
The extent of duodenal mucosal lesion was classified into three categories of bulb only, postbulbitis, and panduodenitis. The presence of H. pylori was detected with any of following methods: (1) rapid urease test; (2) urea breath test; (3) histological examination including H&E staining or Giemsa staining; and (4) serologic test for H. pylori antibody.
The duodenal mucosal lesions were categorized into primary and secondary lesion. Secondary duodenal mucosal lesions were defined as having underlying local or systemic causes; tuberculosis enteritis with/without active pulmonary tuberculosis, cytomegalovirus (CMV), Epstein-Barr virus, fungal, or parasite infection were diagnosed by histological examination including H&E and special stain. For cases of medical history related with duodenal lesions, i.e., IBD including Crohn’s disease or ulcerative colitis, radiation duodenitis, autoimmune diseases such as Henoch-Schonlein purpura (HSP), Bechet’s disease, secondary lesion was defined as having histological findings with clinical manifestations. In these cases, histological re-examination was performed for the diagnostic ascertainment. Primary duodenal mucosal lesion was defined as cases without definitive underlying disease.
Statistics analysis
Descriptive and frequency analysis of the data from the study was expressed as counts, percentage, and mean ± standard deviation. Chi-square test with exact test was used where applicable. Logistic regression was performed for univariate and multivariates analysis to evaluate the predictors for secondary duodenal mucosal lesions. p values of < 0.05 were considered to denote statistical significance. All data were analyzed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patient’s characteristics
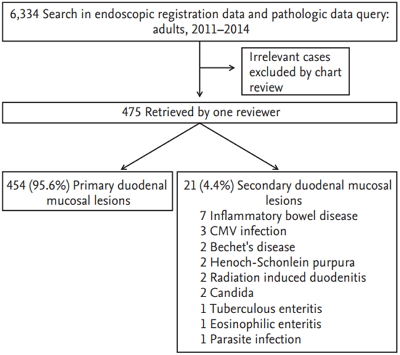
Among 6,334 hospitalized patients have undergone upper endoscopy, 475 patients were diagnosed with endoscopic duodenitis or duodenal ulcers (Table 1). The primary duodenal mucosal lesion was observed in 454 patients (95.6%) and secondary duodenal lesion was detected in 21 patients (4.4%). The most frequent secondary cause was IBD (n = 7), and other causes include CMV infection (n = 3), Bechet’s disease (n = 2), HSP (n = 2), radiation induced duodenitis (n = 2), Candida (n = 2), tuberculosis enteritis (n = 1), eosinophilic enteritis (n = 1), and parasite infection (n = 1) (Fig. 1).
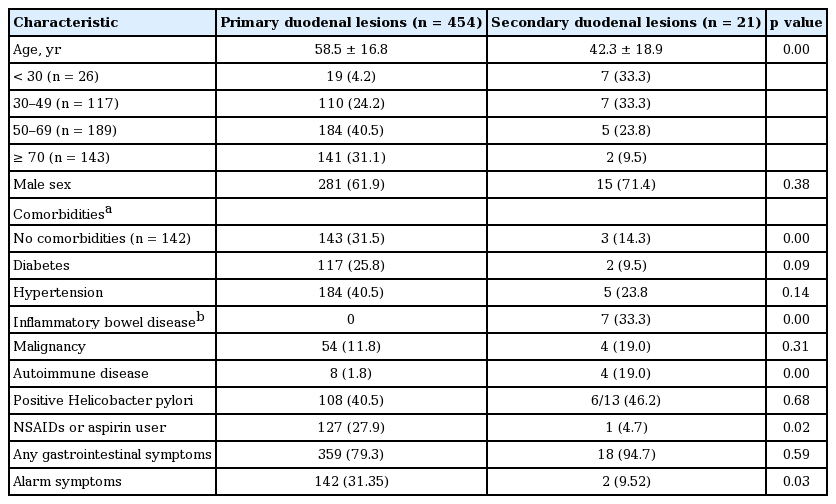
In comparison of characteristics of primary and secondary duodenal lesions, the mean age of secondary group was significantly lower than that of primary group (42.3 ± 18.9 years vs. 58.3 ± 16.8 years, p = 0.000). The proportion of patients under age of 30 in the secondary group was higher than that in the primary group (33.3% vs. 4.2%, p = 0.001). However, there was no significant difference observed by gender. Pulmonary tuberculosis and chronic renal failure are more prevalent in primary group compared to secondary group, but no difference in having diabetes mellitus, hypertension, or malignancy. H. pylori was similarly detected in both primary and secondary group (40.3% vs. 46.2%, p = 0.682). The proportion of usage of NSAIDs or aspirin was significantly higher in the primary group than the secondary one (27.9% vs. 4.7%, p = 0.019).
There were any GI symptoms in 79.4% of primary duodenal lesion and 85.7% of secondary lesion. Especially, abdominal pain was more commonly detected in the secondary lesion compared to primary lesion, but it did not reach the statistical significance (24.0% vs. 57.1%, p = 0.06). The frequency of alarm symptoms such as vomiting or GI bleeding in the primary group was significantly higher than that in the secondary (31.4% vs. 9.52%, p = 0.033).
Difference of endoscopic findings between primary and secondary duodenal lesions
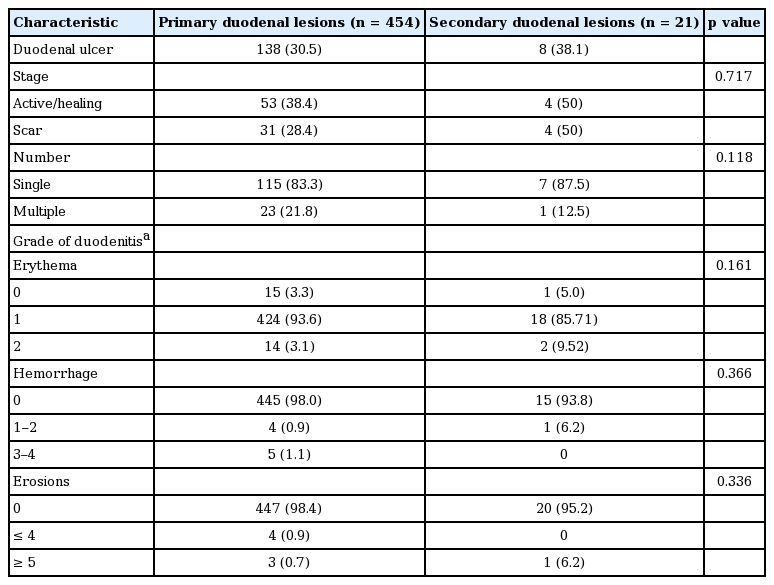
Clinical aspects of endoscopic finding in each patient group indicated significant difference in some respect (Table 2). According to the extent of the lesion, involvement of distal part of duodenum including postbulbitis or panduodenitis was more frequently detected in secondary lesions than in the primary lesions (30.0% vs. 5.2%, p = 0.000) (Fig. 2). Ulcerative lesions, including active or healing stage of ulcer, were similarly detected in primary and secondary lesions (38.4% vs. 50.0%, p = 0.717).

Difference of disease extent of duodenum between primary and secondary duodenal mucosal lesions. Involvement of distal part of duodenum including postbulbitis or panduodenitis is more frequently detected in secondary lesions than in the primary lesions (30.0% vs. 5.2%, p = 0.000).
The severity of duodenitis was measured by the degree of erythematous mucosal changes, number of hemorrhage or erosions (Fig. 3). The erythematous mucosal changes were most frequently accompanied findings in both groups (96.7% vs. 95.2%) and hemorrhages or erosive mucosal lesions were rarely detected in both group. There was no difference of the severity of duodenitis between two groups. In patients with IBD, while various types from active inflammation to chronic inflammation were discovered, mainly nonspecific inflammation was recorded although cryptitis was accompanied in 2/6 patients (Fig. 4). Furthermore, granuloma which implies direct invasion of Crohn’s disease was not observed.

Endoscopic duodenal finding of secondary duodenal lesions. (A) Henoch-Schonlein purpura. (B) Eosinophilic enteritis. Both lesions are detected in second portion with shallow and diffuse mucosal lesion.

Pathologic finding of duodenal biopsy in duodenal lesion with inf lammatory bowel disease patients. Various types from active inf lammation to chronic inf lammation are discovered. (A) Chronic inflammation. Dense lymphoplasmacytic infiltration with a few eosinophils is noted (H&E, ×200). (B) Chronic active inflammation with cryptitis. Acute mixed inflammatory infiltrate is identified (H&E, ×200).
Univariate and multivariate analysis of predictors for secondary duodenal lesions
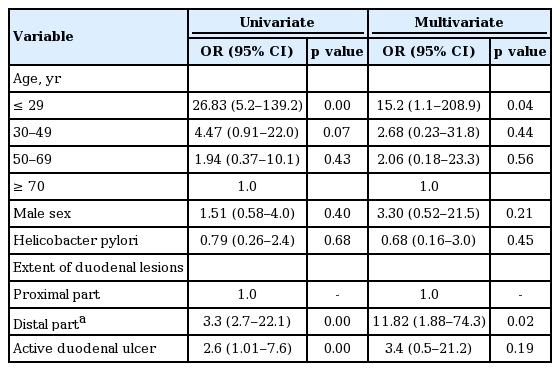
Table 3 shows result of univariate logistic regression analysis used to identify the predictors for secondary duodenal lesions. The young age under 30 years increased the odd for the secondary lesions compared to the elderly (≥ 70 years). The involvement of second portion of duodenum or panduodenitis was significantly related with secondary lesion, and the presence of active duodenal lesions was significantly related with the primary duodenal lesions. By multivariate analysis, young age of 30 years old and the extent of mucosal lesions were significant predictors for the presence of secondary duodenal mucosal lesions.
DISCUSSION
The present study revealed that there is clinical distinction to categorize primary and secondary duodenal mucosal lesions. The majority of patients had normal duodenal mucosa or nonspecific duodenitis; however, in some cases, these duodenal lesions might be related with clinical significance. The secondary duodenal mucosal lesions related with specific pathogenesis was frequently detected in young age group and manifested in distal part of duodenum including panduodenitis or postbulbitis.
There was no significantly different observation made for existence of clinical symptoms between primary and secondary lesions. However, observation of alarm symptom was lower in its frequency in secondary group than that in the primary group while distribution of lesion was higher in regions beyond bulb. This observation implies that duodenal lesion discovered in parts other than bulb by endoscopy can be more related to other specific etiology. The previous study which reviewed pathology and clinical behavior of patients diagnosed with peptic duodenitis in 2nd part, more than 80% of the patients were diagnosed with celiac disease (54.1%) in Western country [21]. It may be difficult to apply this result to our study as case of celiac disease is extremely rare for Koreans. However, it is already known that more cases of duodenal lesion accompanying autoimmune disease such HSP are found in 2nd than in bulb [16,22].
There were several studies in recent with effort to identify the meaning of duodenal lesion in IBD. It is noteworthy that H. pylori infection rate is substantially lower and frequency of duodenitis is higher in IBD patients compared to these in general population [23,24]. In our study, one out of seven IBD patients accompanied duodenal ulcer had H. pylori infection. While the cause of low H. pylori infection rate in IBD has not been clearly identified, it might be suggested with long-term use of sulfasalazine or 5-aminosalicylic acid causing bacterial eradication [24,25]. Granuloma is rarely observed in patients with Crohn’s disease and it is consistent with the previous study [24]. Comparative analysis with other neighboring clinical specimen is important in order to identify focal inflammation sign [25-27]. However, it is difficult to determine duodenal involvement of IBD by histopathological evaluation and H. pylori negative upper GI lesion by routine endoscopy was high in 30% to 70% of Crohn’s disease patients without symptoms [26,28]. There is controversy whether to view these duodenal lesions as Crohn’s disease involvement. However, mononuclear cell infiltration on duodenal mucosal biopsy was more frequently observed in Crohn’s disease and it was related with the increased level of proinflammatory cytokine, such as interleukin 6 [26].
Duodenal mucosal lesion has been generally perceived as peptic disease, and it has been proved in number of studies that H. pylori was more frequently detected in duodenal ulcer patients compared to control group [29,30]. However, in large scale multicenter study in the United States, H. pylori was detected in 40.5% of primary duodenal lesion and 46.2% of secondary duodenal lesion. There showed no significant difference in infection rate between two groups, and the rate was similar to 59.6% of general population [31]. While the prevalence of duodenitis was not significantly different in developing countries, in which H. pylori is noticeably high [4,32]. This result implies that H. pylori infection might not be the only main cause of duodenitis.
The present study suggests that one of the distinctive factors to classify secondary duodenal lesion from primary is age. The incidence rate of duodenal lesion accompanying other disease was significantly higher in the younger age group than the patient group with primary duodenal lesion. A study found that rate of upper GI involvement in IBD has tendency to increase with lower age when other variables are controlled; however, precise cause has not been clearly identified although it can be related to onset age of the disease [25].
There are some potential limitations of this study. This study was conducted with retrospective manner and misclassification might have reduced the secondary duodenal mucosal lesions because duodenal biopsy might not be performed in all cases of endoscopic duodenitis, although we took every step to minimize this issue. Many studies have been conducted to correlate endoscopic finding of duodenitis and pathologic diagnosis; however, conclusion of such studies are divergent [33-37]. It is important to enforce the endoscopy and biopsy together for an accurate diagnosis.
In conclusion, there is a rare overlap with duodenal lesions of different pathogenesis including IBD or autoimmune disorders. However, duodenal involvement of distal part of duodenum or duodenal lesions in young age seems distinctive features of duodenal mucosal lesions associated with other underlying pathophysiology.
KEY MESSAGE
1. Nonspecific duodenitis are frequently detected; however, in some cases, these duodenal lesions are related with clinical significance.
2. Secondary duodenal mucosal lesions associated with specific pathogenesis such as inflammatory bowel disease, cytomegalovirus infection, autoimmune diseases are commonly found in young inpatients and appear as panduodenitis or postbulbitis.
Notes
No potential conflict of interest relevant to this article was reported.