Recent advances in diagnostic technologies in lung cancer
Article information
Abstract
The increase in lung cancer incidence of Korea has been dampened since 2000; however, increased human lifespan, interest in health care and the widespread implementation of health examinations have resulted in a considerable rise in detection of small lesions that need to be differentiated from lung cancer. Detection of lung cancer at an early stage rather than at a symptomatic advanced stage is also increasing, suggesting that there are increasing diagnostic demands for small peripheral lung lesions. The development of new molecular diagnostics, including next generation sequencing, companion diagnostics that accompany development of new anti-cancer drugs, and re-biopsy for application of new therapeutic modality accelerate the development of lung cancer diagnostics. In this review, we extensively describe the current available diagnostic tools in lung cancer.
INTRODUCTION
According to the annual report of the Korean National Cancer Registration Statistics, the crude incidence of lung cancer per 100,000 people in 2016 was 50.4, the 4th highest among all cancers, following that of stomach, colon, and thyroid cancer. Lung cancer incidence appears to have continuously increased from 1997, with 52.7 crude incidence rate per 100,000 people in the year 2017; however, based on the age-adjusted ratio, it has remained steady since 2005, peaking at 28.9 cases per 100,000 people (Fig. 1) [1]. This tendency is slightly different in male versus female population, with lung cancer incidence gradually decreasing for the former but still slowly increasing for the latter; this may be attributed to a decline in male smokers versus a rise in female smokers [2].

Annual incidence (A) of all cancer and lung cancer, and crude incidence rate per 100,000 population (B) of all cancer and lung cancer from 2000 to 2018.
According to the 2018 report on the cases of death from the Statistics Korea, 26.5% of all deaths were cancer-related. Among all cancer-related deaths, the crude mortality rate due to lung cancer reached 34.8 cases per 100,000 people, and it was more than that from other cancer types (Fig. 2) [3,4]. Despite this, the 5-year relative survival rate for lung cancer has improved dramatically from 11.3% in the first survey period (1993 to 1995) to 28.2% (2012 to 2016). However, its survival-related prognosis is still the second worst following that of pancreatic cancer, with an 11.4% 5-year survival rate (survey period: 2012 to 2016). These findings imply that majority of lung cancer patients are diagnosed at advanced stages which cannot be cured by surgery or other therapeutic modalities, but also indicate that there are great demands for improvement in the diagnosis and treatment. Recently, to standardize the diagnosis and treatment of lung cancer, the Korean Health Insurance Review and Assessment (HIRA) has developed indicators, applied evaluation guidelines and reported the results [5]. Moreover, in July 2019, Korean national lung cancer screening projects using lowdose chest computed tomography (CT) were launched for groups at high-risk for lung cancer. In this review, we discuss the diagnostic methods currently widely used for the diagnosis of lung cancer and describe their advantages and limitations.
CLINICAL MANIFESTATIONS
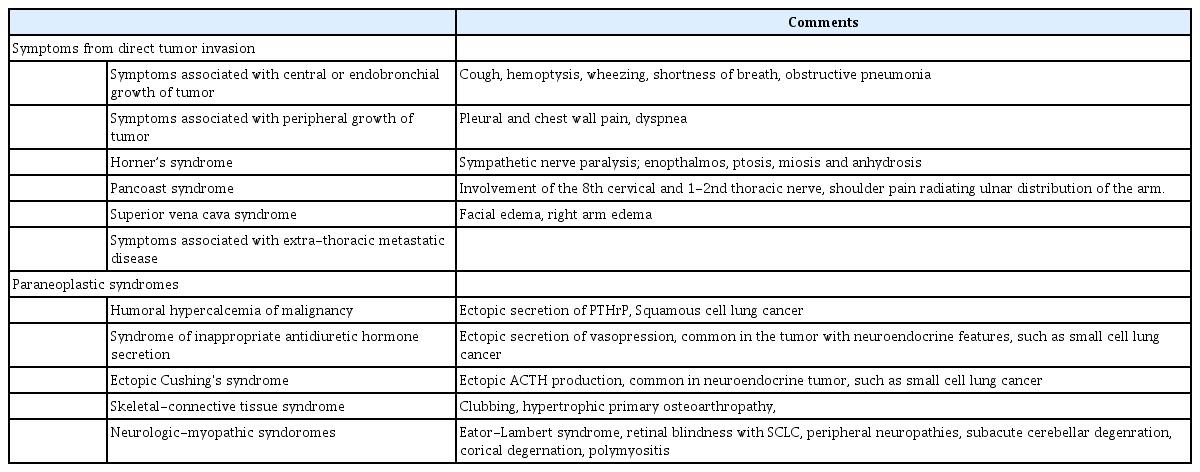
Symptoms of lung cancer can be classified into two categories: caused by the direct mass effect or invasion of the tumor or by paraneoplastic symptoms, which are systemic symptoms that are due to substances secreted by the tumor (Table 1). Symptoms owing to direct tumor invasion include cough, hemoptysis, wheezing, shortness of breath, obstructive pneumonia due to endobronchial growth of the primary tumor, and chest wall pain and shortness of breath due to restrictive dysfunction owing to peripheral growth of the tumor. Regional spread of tumor into the thorax can also cause symptoms depending on the involved organs. Invasion of the trachea by the tumor may result in dyspnea, invasion of the esophagus can cause dysphagia, involvement of the recurrent laryngeal nerve causes hoarseness, and diaphragmatic nerve involvement leads to respiratory distress related to diaphragm paralysis. In addition, invasion of the sympathetic nerves of the cervical spinal cord causes Horner’s syndrome, characterized by enophthalmos, ptosis, miosis, and anhydrosis. Pancoast syndrome, caused by the invasion of the 8th cervical and 1st or 2nd thoracic nerve, presents as shoulder pain radiating along with the ulnar nerve distribution, and invasion of the superior vena cava leads to superior vena cava syndrome [6,7]. Lung cancer can metastasize to any organ in the body, which leads symptoms related to the metastatic lesion.
Paraneoplastic syndromes can occur in case of both benign and malignant tumors but are not correlated to the tumor mass or invasion, and are more commonly observed in tumors with a neuroendocrine origin. They occur in approximately 10% of lung cancer patients. Humoral hypercalcemia of malignancy, syndrome of inappropriate antidiuretic hormone secretion (SIADH), and ectopic Cushing’s syndrome represent the most commonly reported symptoms. It is often challenging to distinguish symptoms caused by metastases of the carcinoma, and inappropriate diagnosis is associated with inappropriate treatment. Therefore, special attention is required when these particular symptoms are noted. Hypercalcemia is a life threatening complication often observed in lung squamous cell carcinoma and is caused by the ectopic secretion of the parathyroid hormone (PTH) or the PTH-related peptide inducing symptoms such as frequent urination, thirst, vomiting, nausea, abdominal pain, constipation, and, in severe cases, loss of consciousness. Hyponatremia in SIADH caused by the ectopic secretion of atrial natriuretic peptide can also occur frequently and requires careful evaluation as it may lead to loss of consciousness or death due to cerebral edema. Small cell lung cancer and bronchial carcinoid tumors may also lead to the development of Cushing’s syndrome caused by an ectopic secretion of the adrenocorticotropic hormone and other hormones that can lead to neurologic symptoms owing to the formation of auto-antibodies [8]. In any case, if symptoms occur, it is likely that the disease is impossible to cure; thus, careful monitoring and accurate diagnoses must be carried out to improve prognosis in these patients. If suspected lung cancer lesions are found in high-risk patients or in previous examinations, it is necessary to accurately identify the nature of the lesion and conduct appropriate tests in a timely fashion, preferably prior to the occurrence of other symptoms.
IMAGING STUDIES
Several imaging modalities can be used for the diagnosis and staging of patients with lung cancer. In real-world practice, combination of several imaging studies is needed to ensure accuracy of the diagnosis.
Chest CT
Chest CT is a fundamental imaging tool for the evaluation of lung cancer and is most commonly used as a noninvasive modality for the screening and staging of lung cancer. It is especially useful to define the size, location, and characterization of lung lesions, which are surrounded by air-filled lung tissue. Additionally, mediastinal and hilar lymphadenopathy, pleural effusion, metastasis in the liver, adrenal glands, bone, and other parts of the thoracic cavity can be assessed by chest CT. Since September 2019, low-dose chest CT (LDCT) has been adopted? for the screening of lung cancer in high-risk individuals in Korea. Screening with LDCT rather than conventional chest radiography is efficient to detect small lung nodules, leads to early intervention, and improves prognosis of lung cancer patients. The U.S. National Lung Cancer Screening Trial demonstrated that LDCT screening leads to a 20% reduction in lung cancer-associated mortality in high-risk populations compared to that seen with chest X-ray. Patients with positive results after LDCT need to be further evaluated using other diagnostic procedures [9].
Positron emission tomography-CT
Positron emission tomography (PET), using uptake of the radiolabeled glucose analogue [18F]-fluoro-2-doxyglucose by metabolically active cells, is a useful test for staging and thus deciding a treatment modality. It is also useful for the detection of extrathoracic disease. However, a false negative result may occur in cases of small lesions less than 1 cm in size and in tumors with low metabolic activity such as in case of carcinoid tumors or bronchioloalveolar cell carcinoma. In contrast, false positives can also occur when inflammatory conditions, such as pneumonia or granulomatous disease, are present. According to a report by Lee et al. [10], the false positive rates for PET-CT in the identification of mediastinal lymph node (L/N) metastasis in Korea can be as high as 87% when using endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) as a gold standard and significantly higher in patients older than 65 years with squamous cell carcinoma or pneumoconiosis. However, the same study reported that current or past history of tuberculosis did not significantly affect the false positive rate, unlike previous reports indicating that the usefulness of PET-CT decreased in areas with a high prevalence of tuberculosis [10].
Brain magnetic resonance imaging
Lung cancer, along with breast and malignant melanoma, are common causes of brain metastases, with lung cancer being the most common. Brain metastases can be detected as an early symptom in approximately 10% of diagnosed patients and associated with significant morbidity and limited survival [11]. The presence of symptomatic brain metastases is an important consideration in the selection of therapeutic agents and treatment modality. As the survival of lung cancer patients increases, improvement of drug permeability of the bloodbrain barrier has become an important consideration for pharmaceutical companies when developing anti-cancer drugs. HIRA guidelines recommend assessment of brain metastases for initial staging and preoperative evaluation of lung cancer [5]. However, with the recent increase in the early diagnosis of lung cancer, the need for brain magnetic resonance imaging (MRI) for staging tumors less than 2 cm in size has been questioned [12].
Whole body bone scintigraphy
Approximately 30% to 40% of non-small cell lung cancer (NSCLC) patients develop bone metastases that lead to poorer prognosis and worsen quality of life during the remaining life. Whole body bone scintigraphy (WBBS) is useful for the detection of bone metastases, when used in conjunction with other relevant clinical findings such as bone pain, elevated alkaline phosphatase, and hypercalcemia. It also has a high false positive rate due to trauma, inflammation and degenerative change of skeletal system and thus, the routine use of WBBS is not recommended by the National Comprehensive Cancer Network guidelines [13]. Moreover, implementation of WBBS for lung cancer is on the decline in Korea as the HIRA indicator does not include bone scans as imaging tools conducted for lung cancer bone metastasis [5].
BIOPSY PROCEDURES
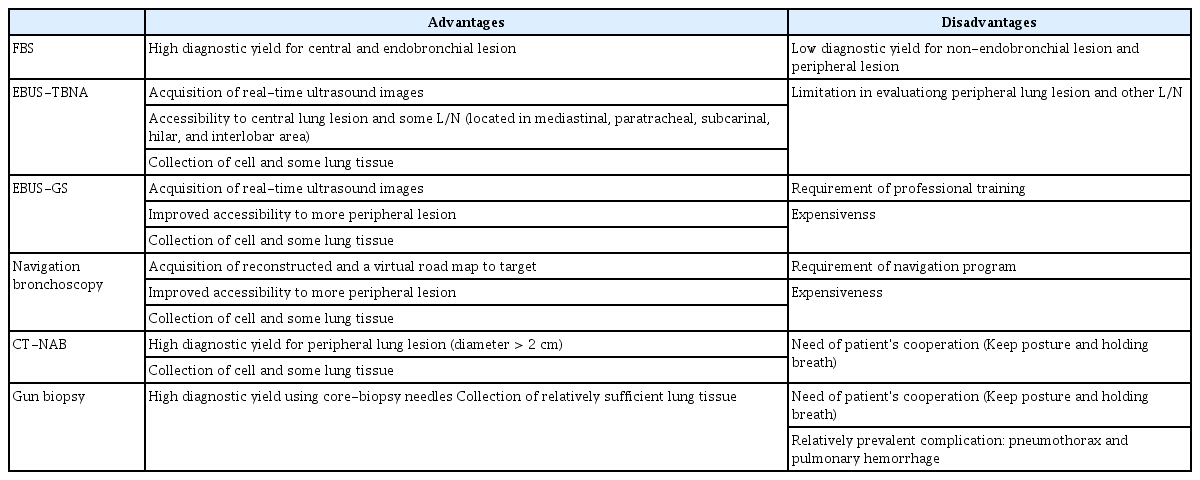
Nowadays, biopsy has become indispensable not only for the diagnosis of lung cancer by confirming the presence of lung cancer cells but also for selection of appropriate treatment by molecular genetic testing. There are several tools for lung tissue biopsy that are currently used for diagnosis and staging of lung cancer. Selection of the diagnostic tools should take into account invasiveness and accuracy but also the location and characteristics of the lesion, the general condition of the patient, and the level of experience of the technician performing the examination (Table 2).
Fiberoptic bronchoscopy
The bronchial pathway to the target lesion and location where the sampling to be performed using forceps and brushes should be determined by careful review of the patient’s anatomy before fiberoptic bronchoscopy (FBS) procedure. The diameter of commercial FBS is around 6 mm and lesions locating up to subsegmental bronchus can be detected and biopsied using FBS. The diagnostic yield for FBS is low, ranging from 20% to 60% [14]. The size and location, and visibility of lesion are important factors that influence the diagnostic yield. Lung lesions less than 2 cm in diameter located in peripheral lung areas typically lead to low yields. In the case of endobronchial lesions that are located in central regions, three or more biopsies are recommended to achieve high diagnostic yields. Transbronchial biopsy, bronchial washing, and bronchial brushing can also improve the diagnostic yield for the lesions that are not directly visible by FBS. For peripheral lung lesions that cannot be reached by routine FBS, fluoroscopy guided transbronchial lung biopsy can increase the accuracy of the diagnosis. Recently, other fusion techniques, including EBUS and navigation bronchoscopy, have been developed and used to improve diagnostic yield.
EBUS-TBNA
EBUS-TBNA, which was introduced to identify mediastinal L/N metastasis of lung cancer, is now widely used not only for the examination of L/N but also for the identification and biopsy of central lesions that cannot be reached by FBS. The EBUS-TBNA needle is typically inserted through the wall of the trachea or main bronchus into the mass or the lymph node. During this procedure, the position of the target lesion is confirmed based on real-time ultrasound images [15]. The highest mediastinal, upper paratracheal, lower paratracheal, subcarinal, hilar, and interlobar L/Ns can be assessed using this methodology with low complication rate [16]. However, the para-aortic, aortopulmonary window or subaortic, paraesophageal, and pulmonary ligament L/Ns are not accessible through this technique. The diagnostic yield for EBUS-TBNA is high with a sensitivity of 90% and a negative predictive value of 93% [17]. In patients with negative results in a standard FBS, EBUS-TBNA was able to confirm primary central lung cancer with a sensitivity of 77% [18].
EBUS using a guide sheath
Using a radial EBUS probe, it is possible to detect peripheral lung lesions surrounding the tip of the probe. The image of radial-type EBUS can be obtained using a guide sheath (GS). Biopsy forceps and bronchial brushes can be placed using GS. This method is superior to FBS or EBUS accessing small peripheral lung lesions [14]. The accessible airways closest to the lesion are marked as targets. The radial probe is then inserted into the target lesion through the bronchoscope. After retraction of the radial probe, forceps or brushes can be placed to further biopsy target lesions. Until recently, the diagnostic yield and safety of EBUS-GS have not been fully evaluated. Most studies investigating this issue were based on single center case reports or were meta-analyses. However, a larger number of studies have now shown the good diagnostic yield of EBUSGS and confirmed that it is a well-tolerated procedure [14,19,20].
Navigation bronchoscopy
Navigation bronchoscopy is generally adopted for small peripheral lung lesions to increase diagnostic accuracy. This technique includes three stages. In the first stage, or the planning stage, a CT scan is reconstructed and a virtual road map to the target is created. In the second stage, or registration stage, an electromagnetic field is created around the body using sensors to overlap the virtual and the actual body. The last stage is the navigation stage where the above information is used for navigation to guide the probe to the target site [21]. The diagnostic yield of this method has been found to range from 33% to 97% [22]. Incidence of pneumothorax is the most frequent complication for this method and occurs in approximately 3% of all cases [23]. The technician’s expertise; the volume, location, and characteristics of the lung lesions; concurrent EBUS; and the use of catheter aspiration are important factors that can influence the diagnostic yield of this method.
Transthoracic needle aspiration biopsy with CT guidance
CT-needle aspiration biopsy (NAB) is one of the most commonly used tools to secure lung tissue, and its diagnostic yield is dependent on the size of the target lung lesion. Typically, lung nodules over 1 to 2 cm in size are good candidates for CT-NAB. Previous studies have shown that the diagnostic yield for CT-NAB ranged from 77% to 94%. It is an invasive procedure and can provide an alternative to surgery for the confirmation of lung cancer diagnosis. However, this technique is associated with a risk of complications. A recent study revealed that the associated rates of pneumothorax and bleeding were 20% and 3%, respectively [24]. Another study showed that pneumothorax requiring intervention occurred in 4.3% of patients, and that major complications developed in 4.4% of those patients [25]. Larger needle diameters, increased transverse lung parenchyma, and smaller lesion sizes are risk factors for the development of major complications in this procedure [25]. Contraindications to the use of CT-NAB are bleeding diathesis, low platelet count, prolonged prothrombin time or partial thromboplastin time and international normalized ratio, pulmonary hypertension, emphysematous disease and chronic obstructive pulmonary disease, large bullae in the biopsy path, and patients with challenges such as intractable cough or those using mechanical ventilation. The ability of patients to hold their breath and maintain their position is important for this procedure. A careful selection of patients is needed in order to attain better yields and reduce complication rates. Recently, improvements have been made to this technique including the addition of laser guidance to improve its accuracy and reduce the risk of complications [26].
Gun biopsy
Various types of needles are available for CT-NAB and the choice of needle is dependent on the size, location, and characteristics of the target lesions, and the technician’s expertise and preference. There are mainly three types of needles used: aspiration needles, cutting needles, and automated core biopsy needles. Aspiration needles are thin-walled and flexible, and cellular material for microscopic or cytological diagnosis can be obtained using these needles [27]. Cutting needles, which are modified aspiration needles with a side cutting tip, are used to obtain histologic specimens. Lastly, core biopsies can be performed using a biopsy gun without causing crushing injuries. Specimens obtained from core biopsies increase the rate of definite benign disease and allow for the characterization of different cell types. The diagnostic yield of core biopsy is superior to that of CT-NAB particularly in benign disease and specimen obtained from core biopsy is more suitable for immunochemical and genetic variation test compared to that from fine needle aspiration. On the other hand, it has an increased associated risk of complications. The reported overall complication rate and major complication rate are 38% and 6%, respectively, for this technique. In a previous study, pneumothorax and pulmonary hemorrhage developed in 25% and 18% of patients, respectively [25]. The choice of biopsy needle for transthoracic needle biopsies should be considered on a case-by-case basis for the improvement of the diagnostic yield, purpose of biopsy, and the reduction in the rate of complications.
LABORATORY TESTS
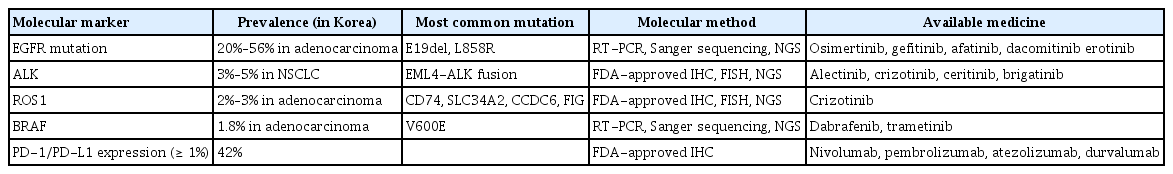
Genomic alterations are an important cause of cancer initiation, growth, and progression. Various technological advancements in cancer genomic analysis platforms have made possible the identification of genomic alterations that can impact the therapeutic response in lung cancer. Therefore, genetics/genomics research is actively being conducted in the lung cancer field, and factors such as EGFR, ALK, and ROS1 have been identified and are currently being used as targets for lung cancer treatment. In addition, data regarding mutations in genes such as BRAF, VEGF, KRAS, RET, and MET are also being used for cancer treatment, in addition to immunological markers such as programmed cell death (PD)-1 and PD ligand 1 (PD-L1) (Table 3) [28].
Polymerase chain reaction based tests
Polymerase chain reaction (PCR) is a revolutionary method invented in 1983 that allows for large amounts of DNA to be amplified in vitro using two primers. PCRbased assays have been continually amended to make it easier to find driver mutations such as EGFR in the medical practice. Dideoxynucleotide sequencing, developed by Sanger et al. [29], of PCR-amplified DNA is the standard method for the detection of genomic mutations; however, it shows suboptimal sensitivity, is labor- intensive, and has long turnover times. Other modified methods, such as PCR-single-strand conformation polymorphism, TaqMan PCR, Cycleave PCR, PCR-restriction length polymorphism, peptide nucleic acid-locked nucleic acid PCR clamp, and mutant-enriched PCR, have been developed and showed improved sensitivity when compared to conventional PCR methods. Kim et al. [30] compared the PNA-mediated PCR clamping method and the direct-sequencing method using the tissues of 112 lung cancer patients. They demonstrated that mutants were identified in 45 samples using the PNA-mediated clamping method, 10-fold more than that when using direct-sequencing, and indicated that this method can be useful for detecting driver mutations [31]. Now, PNA-mediated clamping method is one of the most commonly used methods to detect driver mutations in cancer tissue specimen in Korea.
Next generation sequencing based tests
Next generation sequencing (NGS) is a methodology that quickly decodes a large amount of genome information by breaking down a genome into numerous fragments, reading each fragment simultaneously, and finally combining the data obtained using bioinformatics techniques [32]. Hybrid capture sequencing is used when whole genome, whole exome, or large targeted panels are assessed, whereas amplicon sequencing is used when in-depth reading is required and assay sensitivity is being evaluated. Targeted NGS panels have been validated in several previous studies. Targeted NGS panels, including those for EGFR, KRAS, BRAF, and MET, are widely used in lung cancer [33]. Wider analyses can take up to a week to complete. From these large NGS datasets, in addition to EGFR, KRAS, and BRAF mutations, 36% of lung cancer patients can be found to harbor other potential driver mutations (FGFR, ERBB2, AKT, and MAP2K1, STK11) [34-36]. Many NGS panels that include more diverse genes, tumor mutational burden, microsatellite instability status detection, and fusions are currently under development and will provide more detailed information on lung cancer genetics. Since 2017, the NGS technology base genetic panel test has been designated as an object for national health insurance benefits in Korean lung cancer patients. When applying NGS in lung cancer in Korea and claiming insurance, the essential requirements of the genetic panel should include following 14 genes; HER2, EGFR, ALK, KRAS, NRAS, BRAF, BRCA1, BRCA2, KIT, PDGFRA, IDH1, IDH2, MYC, MYCN [37]. However, NGS can yield false negatives or positives, and therefore, additional tests such as fluorescence in situ hybridization or immunohistochemistry (IHC) for protein overexpression may improve the patients’ diagnosis.
Real-time PCR
There are two types of commonly used real-time PCR methods: real-time PCR using a TaqMan probe and real-time PCR using SYBR Green. Although tissue biopsies are the gold standard for detecting driver mutations, they are invasive and sometimes challenging to obtain due to the patient’s condition and tumor location or size. Contrary to tissue biopsy, a liquid biopsy from plasma, pleural effusion, or bronchoalveolar lavage fluid is typically less invasive [38]. Previous studies have shown promising data using liquid biopsies and real-time PCR [39]. Shin et al. [39] reported a 100% sensitivity and concordance rate of 98.7% with real-time PCR for EGFR using pleural effusions when compared to Sanger sequencing and PNA-mediated PCR clamping.
Transcriptome analysis
In transcriptome analysis, analysis is performed using microarray, an RNA sequencing method that separates mRNA, converts it into cDNA, and analyses its sequence using NGS. Whole transcriptome profiles can easily be obtained from Gene Expression Omnibus (GEO) databases. Lim et al. [40] integrated robust datasets into the bioinformatics pipeline using statistical methods and presented normalized datasets in lung cancer. Bang et al. [41] conducted transcriptome analyses for 10 NSCLC patients and reported that genes related to the cell cycle were highly upregulated in lung cancer. They validated these results using public data available in GEO and The Cancer Genome Atlas (TCGA). MFAP4 and AGER genes were significantly downregulated and the SPP1 gene was upregulated in NSCLC, and these genes were significantly associated with poorer prognoses.
IHC tests
IHC is used in the differential diagnosis of adenocarcinoma and squamous carcinoma (SqCC); neuroendocrine marker identification; driver mutation assessment, including that for EGFR/ALK/ROS1 and PD-L1/PD-1 expression; and the differential diagnosis of lung cancer and mesothelioma [7]. Thyroid transcription factor-1 and napsin A are used as adenocarcinoma markers, and p40, CK5/6, and TP63 can be used as SqCC markers [44]. According to World Health Organization classification, assessment of both neuroendocrine morphology and immunohistochemical expression (chromogranin A, synaptophysin, or CD56) is required for the diagnosis of large cell neuroendocrine carcinoma. For the detection of driver mutations, the U.S. Food and Drug Adminstration (FDA)-approved ALK D5F antibody is used for ALK-rearranged NSCLC as a companion diagnostic tool [45]. However, IHC analysis is not used in EGFR mutation screening due to its low sensitivity. IHC methods for PD-L1/PD-1 varies between providers. The U.S. FDA approved the 22C3 antibody for pembrolizumab, the 28-8 antibody for nivolumab, and SP142 for atezolizumab [46]. Many studies have reported that PD-L1/PD-1 levels correlate to treatment response and prognosis; however, further research will be needed to fully understand these pathways in cancer [47]. Calretinin, BAP1, podoplanin, CK5, CK5/6, and WT1 can be potentially used for the diagnosis of mesothelioma [48].
FUTURE DIAGNOSIS FOR LUNG CANCER
With the continuous development of new therapeutic modalities for lung cancer, the demand for re-biopsy has grown concurrently along with the increase in survival rates. Companion diagnostics, including immunochemical, genetic, and transcriptome analyses, for application of lung cancer therapeutic agent are associated with an increasing demand for a large amount of fresh tissue. However, it is often not feasible to perform additional invasive tests to obtain fresh specimens, especially in patients who have experienced relapses during long-term anti-cancer treatment. Therefore, in addition to the development of safe and convenient techniques for obtaining fresh tissues, sensitive laboratory diagnostic methods that can obtain accurate results with a small amount of specimen should be developed. In this regard, liquid biopsy using blood has drawn much attention, but is only useful in patients with an advanced disease stage and has limitations in those with small tumor burden [49]. The utility of circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) in liquid biopsies has been extensively evaluated. Research on CTCs is waning little by little due to the lack of biomarkers that can accurately identify and secure CTCs except epithelial cell adhesion molecule (EpCAM) and to additional requirement for high efficiency platforms that enrich and enumerate CTCs [50]. On the other hand, the clinical usefulness of ctDNA is gradually increasing due to the development of ctDNA preservation and detection technology.
Also, there have been advances not only in bronchoscopy techniques itself but also in adjuvant therapy administered before and after the procedures. EBUS is now standardized diagnostic tool for lung cancer diagnosis and mediastinal L/N staging and EBUS-GS have been introduced at a number of medical institutions in Korea. EBUS-GS has the advantage of being able to reach peripheral small lung lesions and is useful for obtaining tissues. Diagnostic yield of EBUS-GS brushing cytology alone is not still satisfactory, requiring development of technologies that can increase the diagnostic yield [9]. In contrast, the introduction of navigation bronchoscopy is relatively slow, possibly because it serves the similar purpose as EBUS-GS in examining small peripheral lesions but is cost-intensive and requires additional facilities. The lungs are mostly composed of air-filled loose tissue that makes exact examination of the entire lung structure difficult using MRI or ultrasonography alone. However, novel diagnostic tools are being developed by overcoming the shortcomings of different methods and combining their advantages. One example is EBUS, a combination of ultrasound and optical bronchoscopy, employing navigation of the bronchoscope based on CT image analysis.
CONCLUSIONS
Increasing interest in health, regular check-ups, and development of new treatment modalities has led to increasing demands for procurement of tissue for routine diagnosis but also companion diagnostics. In this review, we discussed the diagnostic methods for lung cancer that are widely used in current medical practice, and we described their advantages and limitations including complementary relation between different diagnostic tools with the aim of contributing to the appropriate selection and application of these techniques in lung cancer patients.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by the National Research Foundation of Korea (grant number NRF-2017R1A2B4009017) awarded to Yoon Soo Chang.