Clinical outcomes of acute upper gastrointestinal bleeding according to the risk indicated by Glasgow-Blatchford risk score-computed tomography score in the emergency room
Article information
Abstract
Background/Aims
Acute upper gastrointestinal (UGI) bleeding is a significant emergency situation with a mortality rate of 2% to 10%. Therefore, initial risk stratification is important for proper management. We aimed to evaluate the role of contrast-enhanced multidetector computed tomography (MDCT) for risk stratification in patients with acute UGI bleeding in the emergency room (ER).
Methods
This retrospective study included patients with UGI bleeding in the ER. Glasgow-Blatchford risk score-computed tomography (GBS-CT) was assessed using a combination of GBS and the MDCT scan scoring system.
Results
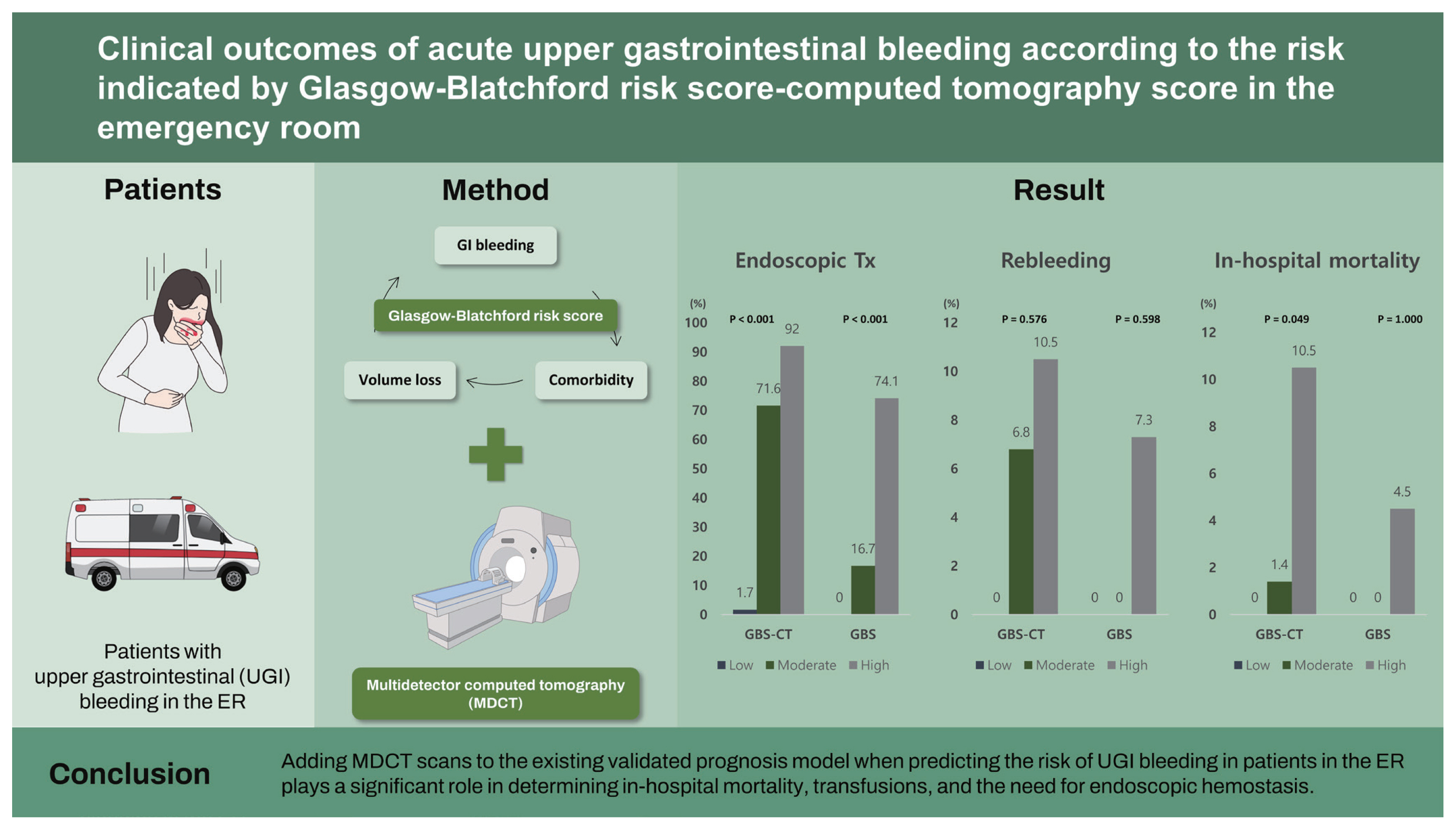
Of the 297 patients with UGI bleeding, 124 (41.8%) underwent abdominal MDCT. Among them, 90.3% were classified as high-risk by GBS, and five patients died (4.0%). Rebleeding occurred in nine patients (7.3%). The high-risk GBS-CT group had significantly higher in-hospital mortality (10.5% in high-risk vs. 1.4% in moderate risk vs. 0% in low-risk, p = 0.049), transfusion amount (p < 0.001), and endoscopic hemostasis (p < 0.001) compared to the moderate- and low-risk groups.
Conclusions
Adding MDCT scans to the existing validated prognosis model when predicting the risk of UGI bleeding in patients in the ER plays a significant role in determining in-hospital mortality, transfusions, and the need for endoscopic hemostasis.
INTRODUCTION
Acute upper gastrointestinal (UGI) bleeding is a significant emergency situation with a mortality rate of 2% to 10% [1,2]. The annual admission rate for UGI bleeding in the United States is estimated to be 160 per 100,000 people, and the estimated direct medical cost of hospital care for patients with UGI bleeding is high [3]. The majority of acute UGI bleeding cases are secondary to non-variceal causes, constituting 80% to 90% of cases. Peptic ulcer disease is the most common cause of UGI bleeding, and in the Unites States and Europe its incidence has decreased in proportion to the reduction in the prevalence of Helicobacter pylori [2]. However, peptic ulcer bleeding remains common and is still a significant medical burden [1]. Additionally, acute variceal bleeding is the most life-threatening complication of liver cirrhosis (LC), and despite recent progress in management, it has a mortality rate of approximately 20% over 6 weeks [4]. Appropriate endoscopic evaluation and management have been shown to improve outcomes, including significantly reducing rebleeding rates, transfusions, surgery, and mortality in patients with UGI bleeding [3].
Determining the severity of UGI bleeding and differentiation of variceal and non-variceal bleeding are important to optimize management, allocate resources efficiently, and identify the disposition of patients. Therefore, initial risk stratification with validated prognostic scales is essential to provide adequate management while minimizing bleeding-related morbidity and mortality. However, in the initial evaluation of the emergency room (ER), it is difficult to know whether there is cirrhosis or variceal bleeding based solely on the patient’s history. Therefore, computed tomography (CT) findings can provide information on diagnosis and predict the need for endoscopic therapy; however, the use of CT in patients with UGI bleeding has received little attention.
The international guidelines recommend the use of scores for risk stratification in acute UGI bleeding, in order to triage patients accurately and aid clinical decisions, such as emergency endoscopic timing, the need for further imaging tests, and the level of treatment required [2,5]. The most widely validated scoring systems for risk stratification of UGI bleeding are the Glasgow-Blatchford risk score (GBS), Rockall score (RS), and AIMS65 score [6–8]. These risk assessment scores were developed to predict clinical outcomes, such as the need for interventions, risk of rebleeding, length of hospital stay, transfusion amount, and mortality. A recent international multicenter prospective cohort study showed that GBS demonstrates high accuracy for prediction of intervention or death compared to the full RS and AIMS65 score [7,9,10]. However, a GBS < 1 was the optimum threshold to predict survival without intervention, and GBS > 7 was the optical cut off to predict endoscopic treatment [7]. In the ER, if a patient with melena shows hemoglobin less than 10 g/dL and the GBS is 7 points, this indicates that the patient is in a high-risk group; however, the risk assessment with GBS tends to classify a significant proportion of patients with UGI bleeding in the ER into the high-risk group.
Contrast-enhanced multidetector computed tomography (MDCT) has been reported to be a potentially useful modality for identification of the origin of bleeding in patients with acute massive GI bleeding [11,12]. As MDCT is commonly used, it can help to rapidly determine a treatment modality. However, the diagnostic accuracy of MDCT before urgent endoscopy in patients with acute UGI bleeding is controversial [11,13–15].
This study was conducted to evaluate the feasibility of additional abdominal MDCT and GBS for initial risk stratification in patients with UGI bleeding in the ER.
METHODS
Study subjects
This historical cohort included adults (age, 18 to 100 years) who were admitted through the ER of the referral hospital due to UGI bleeding from February 1, 2018 to February 1, 2019. Potential cases were identified as having one or more of the following symptomatic, diagnostic, or intervention terms: melena, hematochezia, hematemesis, gastrointestinal (GI) bleeding, ulcer with bleeding, erosion with bleeding, malignancy with bleeding, hemorrhagic gastritis, Mallory-Weiss syndrome, variceal bleeding, endoscopic bleeding control, angiography, embolization and surgery. Of these, adult patients aged 19 years or more who had definite variceal and non-variceal UGI bleeding and were admitted through the ER were included in the study. Exclusion criteria were as follows: lower gastrointestinal (LGI) bleeding or obscure GI bleeding, no endoscopy, no hospitalization, and incomplete medical records, including when there was no bleeding. Three gastroenterologists (T.O.K., E.S.J., and H.A.L.) performed detailed chart reviews to improve the specificity.
A rapid and comprehensive MDCT evaluation can quickly detect GI bleeding and abnormalities in the extra-intestinal structures, which can affect clinical decision-making. To investigate the usefulness of emergency MDCT in the ER during the study period, we compared the risk prediction accuracy of scoring systems, such as GBS, AIMS65 score, and RS. The additional CT score added to the original score was set, and the usefulness of MDCT was evaluated based on the clinical results.
The study protocol was approved by the Institutional Review Board of Ewha Woman’s University (IRB FILE No: 2020-07-040). The requirement for informed consent was waived because we used unidentified data collected from routine medical care.
Data collection
Patient data including age, sex, medical history, symptoms of visiting emergency centers (hematemesis, melena, hematochezia, syncope, dizziness or altered mentality), vital signs (heart rate, systolic blood pressure), mental status, and medications that contribute to bleeding including non-steroidal anti-inflammatory drugs, antiplatelet agents (low-dose aspirin, ticlopidine and/or clopidogrel) and/or anticoagulant agents (warfarin or dabigatran) were collected by electronic medical record review. Laboratory findings included hemoglobin, creatinine, blood urea nitrogen, prothrombin time, international normalized ratio (INR), total protein, and albumin.
Comorbid diseases, such as cardiovascular diseases, diabetes, malignancies, or others, were identified through medical chart review. Hepatic disease was defined as a known history, or clinical and laboratory evidence of chronic or acute liver disease. Heart failure was defined as a known history or clinical and echocardiographic evidence of cardiac failure [6]. Altered mental status was defined as a Glasgow Coma Scale score < 14 or physician-charted designation of disorientation, lethargy, stupor, or coma [8,16]. Whether the patient underwent blood transfusion, and endoscopic hemostasis were also recorded. Indications for blood transfusion were determined by the physician in charge of each patient and included conditions, such as the presence or absence of altered mental status, tachycardia, change in hemoglobin level, systolic blood pressure, and comorbidities.
Scoring system for risk stratification of UGI bleeding
The GBS, AIMS65 score, and RS at the initial endoscopy were calculated for all patients. GBS is a formal risk assessment tool for UGI bleeding, and uses the patient’s blood results, blood pressure, known history, and presentation findings to identify how urgently a patient requires endoscopic therapy [6]. The AIMS65 score is a newer and simpler system (albumin, INR > 1.5, altered mental status, systolic blood pressure < 90, age 65) derived from a much larger population database, and is designed to predict mortality [8]. The RS is calculated using the composite score of the presence of shock, comorbidities, endoscopic signs of bleeding, and diagnosis [17]. High-risk patients were defined as those with GBS ≥ 7, those with RS ≥ 4, and those with AIMS65 score ≥ 2.5 [9,10].
CT was performed in patients with UGI bleeding in the ER in the following circumstances: (1) suspected variceal bleeding; (2) active GI bleeding suspected due to hematemesis, melena, or hematochezia occurring with 24 hours before CT; (3) after stabilization when patients are in a hemodynamically unstable condition. Under these circumstances, CT was performed in patients who had a normal range of kidney function (estimated glomerular filtration rate 60 mL/min/1.73 m2 or higher) and patients with low renal function who agreed to perform CT.
We defined the Glasgow-Blatchford risk score-computed tomography (GBS-CT), which was assessed by a composite of GBS and CT grading, and classified patients into three groups, namely high-risk, moderate risk, and low-risk patients. The high-risk group was defined as ‘having varix, liver cirrhosis, or active bleeding evidence on CT scan’ and ‘patients with 7 or more GBS.’ The moderate risk group was defined as satisfying either criterion. The low-risk group was defined as neither of these criteria; a GBS score of 6 or less, and no active bleeding on CT, varix, and LC.
Management strategies of GI bleeding
Patients were managed according to our emergency bleeding protocols. All patients with non-variceal bleeding received an intravenous proton pump inhibitor infusion before upper endoscopy. Patients with variceal bleeding received an intravenous analogue of vasopressin and antibiotics at the ER.
Endoscopy was performed by specialists within the first 24-hour. Endoscopic therapies were applied based on the following findings: (1) peptic ulcers, Forrest type Ia, type Ib, type IIa; (2) Dieulafoy’s lesion; (3) angiodysplasia; (4) esophageal or gastric varix; and (5) any lesions with active bleeding. Endoscopic therapies included injection of epinephrine or pure ethanol, hemoclipping, argon plasma coagulation and endoscopic band ligation. The door-to-endoscopy time was defined as the amount of time (in minutes) taken from the time of arrival at the ER to the start of the endoscopy. Forrest type IIb, type IIc, type III, or lesions with no active bleeding stigmata were treated with medication without endoscopic therapy.
Angiographic control of GI bleeding is indicated for patients with UGI or LGI bleeding who fail to respond to medical and/or endoscopic therapy. In patients with UGI bleeding, angiographic control of bleeding is generally considered if endoscopic attempts at therapy have failed. The indications for embolization include the following: (1) massive UGI bleeding (transfusion requirement of 4 units of blood or more in 24 hours) or hemodynamic instability (hypotension with systolic blood pressure lower than 90 mmHg and heart rate of ≥ 100 beats per minute) that fails to respond to conservative medical therapy or endoscopic control; (2) active GI bleeding demonstrated by nuclear scintigraphy or computed tomographic angiography examinations; (3) bleeding into pancreatic pseudocysts or visceral artery aneurysms; and (4) hemobilia [18–20].
Surgery as salvage therapy was performed when embolization was unsuccessful, endoscopic therapy failed, or perforation was observed on MDCT scan [8,21].
If hemoglobin was less than 8 g/dL, or if hemoglobin was more than 8 g/dL, the patient’s life condition was unstable, or if there was a massive bleeding (more than 4 units of blood needed within 24 hours), a blood transfusion was required.
Statistical analysis
We compared the proportion using Pearson chi-square test and Fisher’s exact test, and performed an unpaired samples t test for continuous data with mean and standard deviation (SD) between the CT and non-CT groups. Non-parametric variables were compared using the Mann-Whitney U test and Kruskal-Wallis equality of populations rank test. Data were analysed using SPSS Statistics for Windows version 23.0 (IBM Corp., Armonk, NY, USA). All statistical analysis were two-tailed, and statistical significance was set at p < 0.05. All confidence intervals (CIs) were described as two-sided binomial 95% CIs.
RESULTS
Patient characteristics
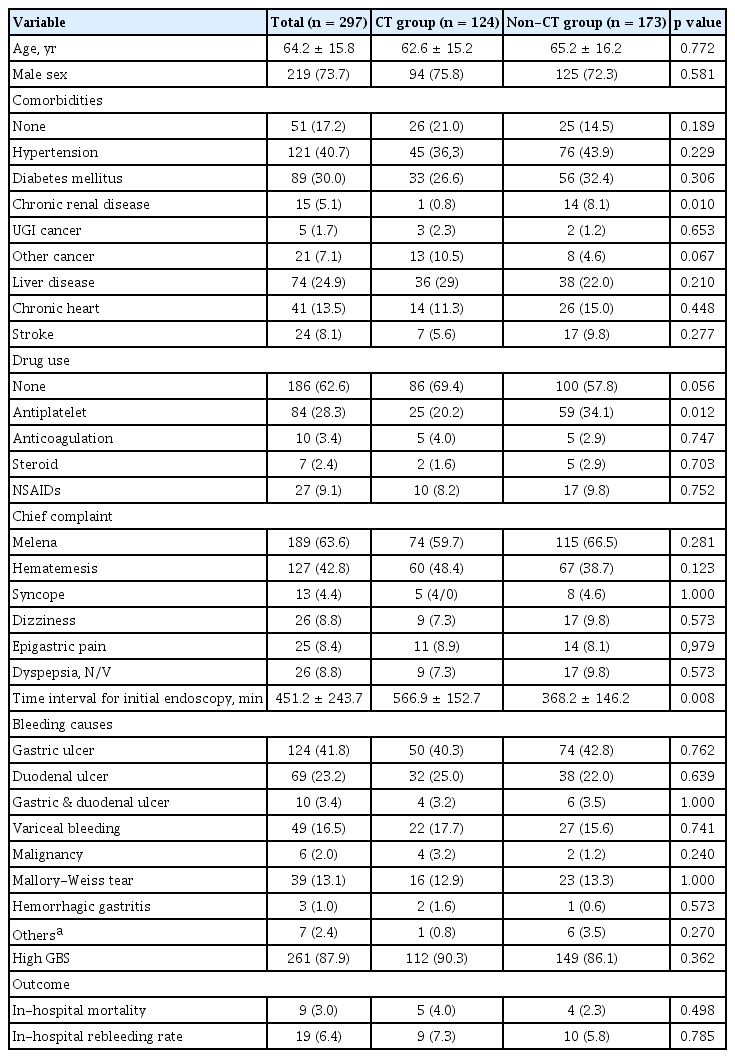
A total of 509 patients who visited the ER for GI bleeding from February 2018 to February 2019 were reviewed initially. We excluded 212 patients, including 99 patients with LGI bleeding; 56 who refused proper management and were discharged on the same day as ER admission, 40 who had no admission and 17 who did not have evidence of bleeding or had insufficient of data. A total of 297 patients were included in the analysis. The mean ± SD of age was 64.2 ± 15.8 years old, with a range of 18 to 100 years old; 73.7% of the included patients were men. Among the patients, 246 (82.8%) had comorbidities and 91 (30.6%) were taking antiplatelet agents (n = 84) or anticoagulant medications (n = 10) on admission. The most common symptom in the ER was melena (63.6%), followed by hematemesis (42.8%), dyspepsia/nausea/vomiting (8.8%), dizziness (8.8%), epigastric pain (8.4%), and syncope (4.4%). The most common causes of bleeding were gastric ulcer (41.8%), duodenal ulcer (23.2%), gastric and duodenal ulcers (3.4%), variceal bleeding (16.5%; esophageal varix, n = 42; gastric varix, n = 7), Mallory-Weiss tear (13.1%) and malignancy (2.0%; esophageal cancer, n = 2; gastric cancer, n = 4).
Nine of the 297 patients (3.0%) died. Their median age was 62.6 years (range, 48 to 92). The causes of death were complications due to hepatorenal syndrome (n = 4), uncontrolled bleeding (n = 2), sepsis due to pneumonia (n = 2), and uncontrolled bacteremia (n = 1). Seven patients had evidence of chronic liver disease on CT and/or endoscopic findings, and one had chronic renal failure.
To compare GBS and GBS-CT, we selected patients who underwent CT (n = 124) in the ER. There were no significant differences in demographics between the CT and non-CT groups except for the presence of chronic kidney disease (0.8% vs. 8.1%, p = 0.010) and the administration of antiplatelet drugs (20.2% vs. 34.1%, p = 0.012) (Table 1). The door-to-endoscopy time, which was time interval from ER visit to initial endoscopy, was longer in the CT group than the non-CT group (566.8 ± 152.7 minutes vs. 368.2 ± 146.2 minutes, p = 0.008).
Prediction of in-hospital mortality with GBSCT grading
Five of the 124 (4.0%) patients in the CT group died. Their median age was 56 years (range, 48 to 67). The causes of death were complications due to uncontrolled bleeding (n = 2), hepatorenal syndrome (n = 1), sepsis due to pneumonia (n = 1), and uncontrolled bacteremia (n = 1). With regards to the two deaths due to uncontrolled bleeding, one patient underwent angiographic embolization and one patient underwent angiography followed by surgery. Four patients had evidence of chronic liver disease on CT and/or endoscopic findings.
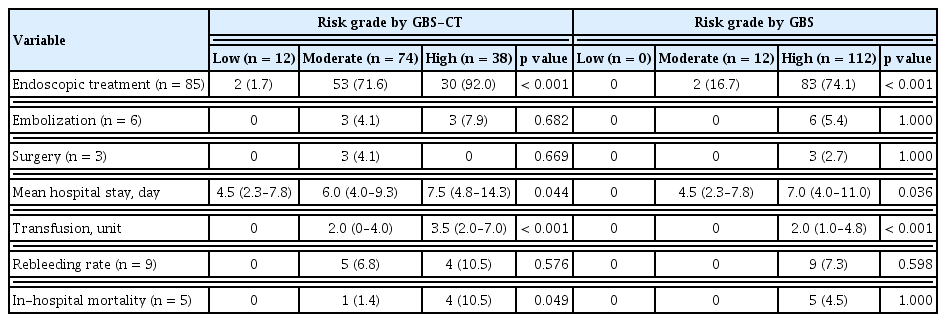
Mortality increased with higher GBS-CT in the CT group, but we were unable to accurately predict mortality with GBS only in the patient group. Mortality was observed in 0/12 (0%) patients at low-risk of GBS-CT, 1/74 (6.8%) at moderate risk of GBS-CT, and 4/38 (10.5%) patients at high-risk of GBS-CT (p = 0.049) (Fig. 1). These trends were found to be statistically significant (p = 0.026) by the linear-by-linear association method. However, according to GBS grading, mortality cases were noted in the high-risk group (4.5%), but there was no difference between the low- and moderate-risk groups (p > 0.99) (Table 2 and Fig. 1).

Prediction of outcomes of upper gastrointestinal bleeding by Glasgow-Blatchford risk score-computed tomography (GBS-CT) and Glasgow-Blatchford score (GBS). (A) Endoscopic treatment. (B) Rebleeding. (C) In-hospital mortality.
Prediction of secondary clinical outcomes with GBS-CT grading
Rebleeding
In-hospital rebleeding occurred in nine patients in the CT group. The GBS score and GBS-CT did not show significant differences in in-hospital rebleeding (Table 1 and Fig. 1).
Endoscopic intervention
Endoscopic treatment was performed in 85 patients (68.5%). According to the GBS-CT grading, 30 (92.0%) of the high-risk patients underwent endoscopic treatment, and 83 (74.1%) of the high GBS group were managed with endoscopic hemostasis. Among patients with a moderate risk of GBS-CT, a higher proportion were managed with endoscopic modalities, compared to the proportion among those with a moderate risk of GBS (71.6% vs. 16.7%). The proportion of patients who were managed with endoscopic treatment among the risk groups showed a statistically significant difference in both scoring systems (p < 0.001) (Table 2 and Fig. 1).
Mean time of hospital stay
The mean hospitalization time was 6.0 days (interquartile range, 4 to 8.5). There was a significant difference between risk groups in the predictive power of both GBS-CT (low-risk 4.5 [95% CI, 2.3 to 7.8] vs. moderate-risk 6.0 [95% CI, 4.0 to 9.3] vs. high-risk 7.5 [95% CI, 4.8 to 14.3], p = 0.044) and GBS score for hospital stay (0.0 vs. 4.5 [95% CI, 2.3 to 7.8] vs. 7.0 [95% CI, 4.0 to 11.0], p = 0.036) (Table 2).
Transfusion requirements
Transfusion was required in 87 patients (65%), and the median transfusion was 2.0 units (interquartile range, 0 to 4). Transfusion amounts according to the GBS-CT grading were significantly different between the three groups (p < 0.001) (Table 2). However, the GBS score was insufficient to demonstrate this trend, as there were no blood transfusions in the low- and moderate-risk groups.
DISCUSSION
Early identification of patients with acute UGI bleeding who are likely to require interventions or to have high mortality can improve the efficiency of critical care and clinical outcomes. In addition, meta-analysis shows that initial massive blood transfusions can induce bleeding and may be related to increased GI bleeding-related mortality [22]. The present study demonstrated that the new scoring system, GBS-CT scoring, which combines abdominal CT findings and GBS, better predicts the risk of UGI bleeding patients and is more effective in determining early endoscopy in the ER. Emergency endoscopy is an important strategy for both the diagnosis and treatment of UGI bleeding. Endoscopy can provide interventions that classify the type of bleeding and hemostasis if necessary [23,24]. Early endoscopic examinations performed within 24 hours of admission are associated with a reduced need for blood transfusions and a shorter hospital stay for patients at high-risk of non-variceal UGI bleeding [5,25]. Combined treatment with vasoactive drugs and endoscopic treatment is the standard care for patients with variceal bleeding [4]. As noted in a retrospective evaluation by Yavorski et al. [26], early endoscopic examination may also reduce mortality. However, emergency endoscopy is not available in many hospitals due to the cost and fatigue of the medical staff.
Several risk scoring systems have been developed to divide patients with UGI bleeding into high- and low-risk categories [5]. However, RS has some limitations in terms of facilitating early decisions around urgent interventions in the management of patients with UGI bleeding, since it requires endoscopic findings. It predicts mortality better than chance alone, but overall should be interpreted with caution; a score of ‘0’ in some studies suggested very low mortality, but in others this was not a consistent indicator [17]. Moreover, when GBS was applied here, most patients with UGI bleeding (> 90%) were allocated to the high-risk group; therefore, proper stratification is likely to be difficult.
Several studies have shown that CT is beneficial in patients with acute lower GI bleeding [27] and acute abdominal pain [28]. Multiphase CT enterography has been proven to be an effective tool for suspected small bowel bleeding in an outpatient setting [22]. Insufficient data on the benefits of CT in terms of patient treatment and outcomes have not been considered in the initial diagnostic form for assessing acute GI bleeding in the ER [29].
Recently, the introduction of MDCT has led to increased image resolution and decreased scanning time [13], and this may be a useful modality for detecting bleeding foci in patients with massive GI bleeding. A previous study showed that MDCT had a moderate diagnostic accuracy in patients with non-variceal bleeding (50.2%); however, it had a diagnostic accuracy of 96.4% in patients with variceal bleeding [13]. Therefore, we evaluated the risk by adding CT scores to GBS, whereby 90% of patients who come to the ER from UGI bleeding are classified as high-risk, which we define as ‘evidence of active bleeding on MDCT, having liver cirrhosis or varix.’ Analysis of risk from GBS-CT showed a significant difference in transfusion amount according to the risk groups; however, the GBS score alone was insufficient to show this trend, as there was no blood transfusion in the low- and moderate risk groups. Door-to-endoscopy time indicated that it is not efficient to perform an emergency endoscope unconditionally early. A cohort study revealed that peptic ulcer bleeding is hemodynamically stable and that delaying endoscopy within 12 hours has a survival advantage over fast endoscopy in patients, and a 6-hour delay was recommended for patients with hemodynamic instability [30]. Emergency CT scans in UGI bleeding patients can determine whether an emergency endoscope should be performed; for example, if emergency CT shows active bleeding or LC, immediate endoscopy is desirable. Analysis of the deaths revealed that seven out of the nine total deaths had cirrhosis, and five of them died of hemorrhage or hepatorenal syndrome. As mortality from UGI bleeding was mainly associated with liver disease, we found a significant difference in mortality prediction when CT findings, including liver disease, were included in the GBS-CT. In general, the increased risk of morbidity due to acute variceal bleeding in adult patients is well documented. Patients with liver disease with high Childs-Turcotte-Pugh scores or decompensated cirrhosis are known to have an increased risk of both morbidity and mortality after GI bleeding [31]. Since the rapid use of early antibiotics and vasoconstriction is directly linked to the patient’s prognosis, it is possible to detect underlying liver disease early through CT and proceed with treatment based on the findings [31]. Moreover, applying GBS-CT further distributes patients to the moderate risk group. Thus, GBSCT can be more meaningful with regards to predicting the need for endoscopic treatment.
There are some limitations to our study. First, this was a retrospective study, which implies a potential information bias due to the study design; therefore, the findings should be interpreted prudently. Seventeen patients were excluded from our initial query due to incomplete data, which might have caused potential bias in the interpretation of the generalization of the present study findings. Second, we set a scoring system that included CT findings. Therefore, patients without CT were excluded from the main clinical outcomes. This can be attributed to selection bias. However, there was no significant difference between the CT and non-CT groups, except for chronic renal failure. In addition, this was a single-center study, and the results may not be applicable to other medical centers. Consequently, other prospective multicenter studies are needed. Lastly, we did not analyse the cost-effectiveness of using a CT scan. For patients who visit the ER due to UGI bleeding, CT scan use results in a higher cost and radiation hazard. However, a significant number of patients with UGI bleeding are likely to undergo imaging tests, including CT scans, after hospitalization to identify underlying liver or pancreatic diseases.
In conclusion, we investigated the additional role of CT scan in GBS, an existing validated prognosis model, when predicting the risk of UGI bleeding in patients with ER. The GBS-CT scoring system showed higher predictability of in-hospital mortality and the amount of blood transfusion required, compared to GBS alone. Furthermore, GBS-CT can help determine whether early endoscopy should be performed.
KEY MESSAGE
1. This study showed that assessing risk using computed tomography (CT) findings with Glasgow-Blatchford risk score (GBS) can help predict in-hospital mortality of patients with upper gastrointestinal (UGI) bleeding in the emergency room.
2. Of the CT results, the presence of active bleeding, gastroesophageal varices, and liver cirrhosis are associated with high mortality in patient of UGI bleeding.
3. This is the first study to evaluate the risk prediction in upper gastrointestinal bleeding by combining emergency CT findings and GBS.
Notes
No potential conflict of interest relevant to this article was reported.