Endoscopic features of cytomegalovirus disease of the upper gastrointestinal tract between transplant and non-transplant patients
Article information
Abstract
Background/Aims
Cytomegalovirus (CMV) disease in the upper gastrointestinal (UGI) tract frequently occurs in immunocompromised patients. However, data regarding UGI CMV disease in non-transplant patients compared with those in transplant recipients are limited. Therefore, we compared the clinical characteristics, endoscopic findings, and outcomes of UGI CMV disease in non-transplant patients with those in transplant recipients.
Methods
We reviewed the medical records of patients diagnosed with UGI CMV disease between May 1999 and January 2022. UGI CMV disease was defined as symptoms or signs of gastrointestinal disease with typical findings of CMV inclusion body and positive immunochemistry stain or CMV polymerase chain reaction from the endoscopic biopsy specimen.
Results
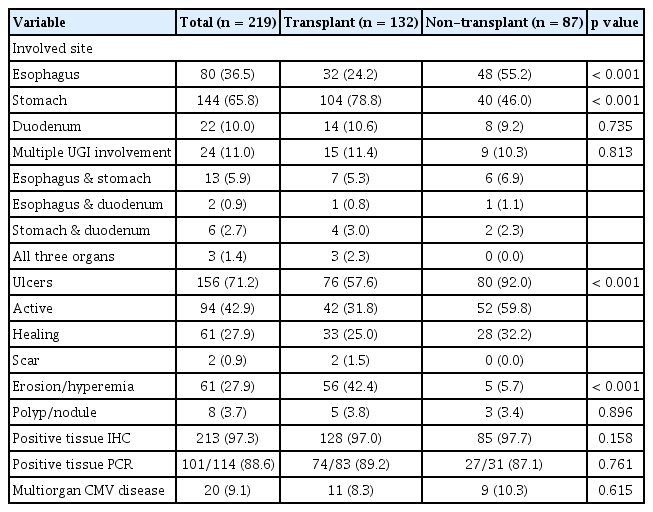
Among the 219 eligible patients, 132 (60.3%) were transplant patients. Age, male sex, and Charlson Comorbidity Index were significantly higher in the non-transplant group than in the transplant group. The most common symptoms were pain and odynophagia (43.8%). Transplant recipients more frequently experienced UGI CMV disease in the stomach than non-transplant patients, typically presenting as erosions or mucosal hyperemia. However, non-transplant patients more commonly experienced UGI CMV disease in the esophagus than transplant recipients, typically presenting as ulcers. The transplant group had a significantly higher clinical response than the non-transplant group.
Conclusions
UGI CMV disease in transplant patients can be present in the stomach in various forms, including ulcers or erosions. In transplant patients suspected of UGI CMV disease, conducting an esophagogastroduodenoscopy with tissue biopsy in any area where even the slightest mucosal abnormality is observed is essential to facilitate a prompt diagnosis.
INTRODUCTION
Cytomegalovirus (CMV), a double-stranded DNA virus that belongs to the human herpesvirus, is one of the globally widespread viruses [1]. In immunocompetent patients, the prevalence of CMV infection in adulthood is > 60%, with the majority of cases persisting as latent infections after an asymptomatic primary infection, followed by reactivation [2,3]. In particular, gastrointestinal (GI) CMV disease is the most common type of tissue-invasive CMV disease, which accounts for 30% of such cases among immunocompetent patients [4].
GI CMV disease has its highest incidence between 30 and 90 days after transplant in patients undergoing transplant and is known to have a major impact on patient morbidity and mortality [2]. Approximately 10% of all transplant patients experience GI CMV disease, with the esophagus being the second most commonly affected site after the colon [5]. Erosions, ulcerations, hemorrhage, GI dysmotility, and, very infrequently, perforations are the possible pathological findings of CMV disease, which can manifest in various forms. However, because of the limited investigations on CMV disease affecting the entire upper gastrointestinal (UGI) tract, diagnosis is difficult, and treatment is usually delayed. In addition, with the recent increase in the number of patients with various forms of chemotherapy-induced immunosuppression, long-term administration of steroids, and human immunodeficiency virus (HIV) infections, CMV infection has become a problem not only for transplant recipients but also for such immunocompromised patients. However, transplant recipients, despite being vulnerable to infections due to long-term immunosuppressive therapy after transplantation, show a very high long-term survival rate, differentiating them from other immunocompromised patients [6,7].
Therefore, we aimed to determine the characteristics of UGI CMV disease manifestation in immunocompromised patients, including transplantation recipients, patients who underwent chemotherapy, those with long-term steroid use, and those with HIV infection, and to determine whether clinical differences exist between transplant and non-transplant patients.
METHODS
Patients
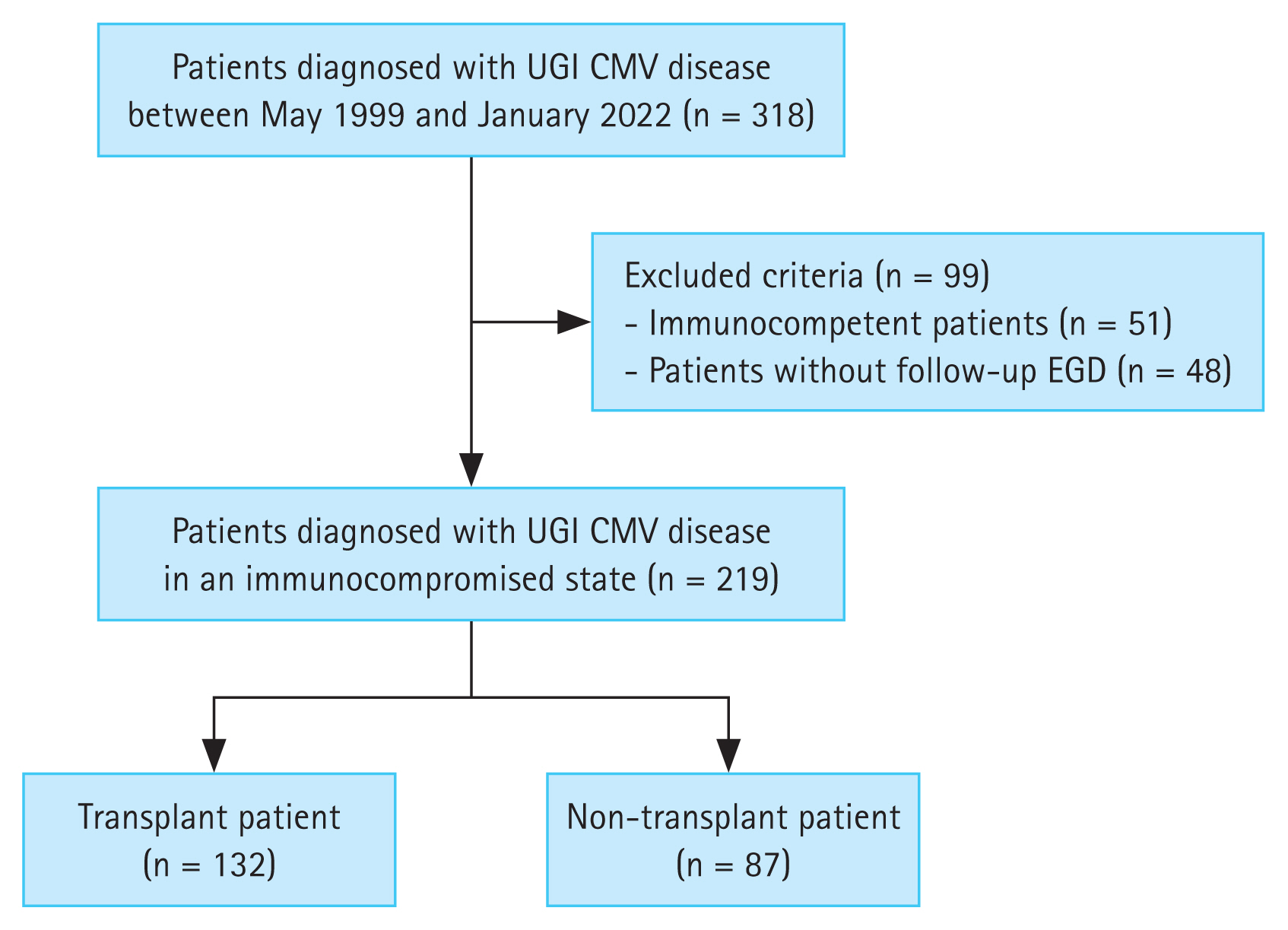
This single-center, retrospective study was conducted at Asan Medical Center in Korea. A total of 318 patients were diagnosed with UGI CMV disease in their esophagogastroduodenoscopy (EGD) examination between May 1999 to January 2022. Among them, immunocompetent patients (n = 51) and patients who did not undergo follow-up endoscopy (n = 48) were excluded from the study. Ultimately, 219 patients were enrolled in this study. Figure 1 illustrates the flowchart of patient inclusion.
Definition
Immunocompromised patients were classified into transplant and non-transplant patients. Transplant patients included both solid organ transplantation recipients, such as liver, heart, and kidney transplant recipients, and patients who underwent hematopoietic transplantation, including allogeneic and autologous stem cell transplantation. In contrast, non-transplant patients included those who underwent chemotherapy or long-term steroid administration (> 20 mg of prednisolone per day for at least 1 month) and those with HIV infection at the point of diagnosis. Chemotherapy patients were defined as the patients who were receiving systemic cytotoxic drugs for hematologic malignancies or solid tumors, as well as those who were undergoing treatment with a combination of cytotoxic drugs and radiation therapy.
UGI CMV disease was defined as the histological confirmation of CMV infection by a positive result of immunohistochemical (IHC) staining or polymerase chain reaction (PCR) of CMV from a biopsy specimen obtained via EGD [8]. Ulcers were classified based on endoscopic findings and categorized into active, healing, and scar stages according to the Sakita–Miwa classification [9]. Multiple UGI involvement was defined as CMV disease diagnosed in two or more UGI organs. In addition, multiorgan CMV disease was defined as CMV disease involving two or more organs, including the UGI tract and other sites, such as the lower GI tract, retina, and lungs. CMV antigenemia was defined as a positive result from the CMV antigenemia assay using the C10/C11 monoclonal antibody (Biotest, Dreieich, Germany), while CMV viremia was defined as a positive result from real-time PCR (Biocore, Seoul, Korea) in blood samples.
Clinical response was assessed through visual confirmation of mucosal lesions via EGD and classified into the following three groups: complete response, partial response, and non-response. Complete response was defined as an improvement of the baseline UGI lesion by > 90%, while partial response was defined as mucosal healing of < 90% compared with the baseline status. Non-response was defined when the lesion remained unchanged or worsened or a new mucosal lesion was observed. CMV relapse was defined as the reappearance of CMV antigenemia/viremia or UGI CMV disease after achieving complete remission following antiviral therapy, while the patient was under regular follow-up.
Data collection
Patients’ baseline medical information, such as age, sex, and symptoms, was retrospectively collected from their medical records. The Charlson Comorbidity Index (CCI) was calculated to evaluate the underlying disease status and comorbidities [10]. In addition, endoscopic data, including the endoscopic features of UGI CMV disease and histopathological reports of the biopsied specimens, were also gathered.
Statistical analysis
Quantitative data, including age, CCI, and duration of follow-up, were expressed as medians with interquartile ranges (IQRs). Numbers and proportions were used to express qualitative data, such as sex, symptoms, CMV infection site, and ulcer stages. Statistical significance was considered at p < 0.05. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 21.0 (IBM Corp., Armonk, NY, USA).
Ethics approval
Ethics approval for data collection was granted by the Institutional Review Board of Asan Medical Center (approval number: 2022–1358), and the need for obtaining informed consent from the patients was waived since the data were collected retrospectively.
RESULTS
Baseline characteristics
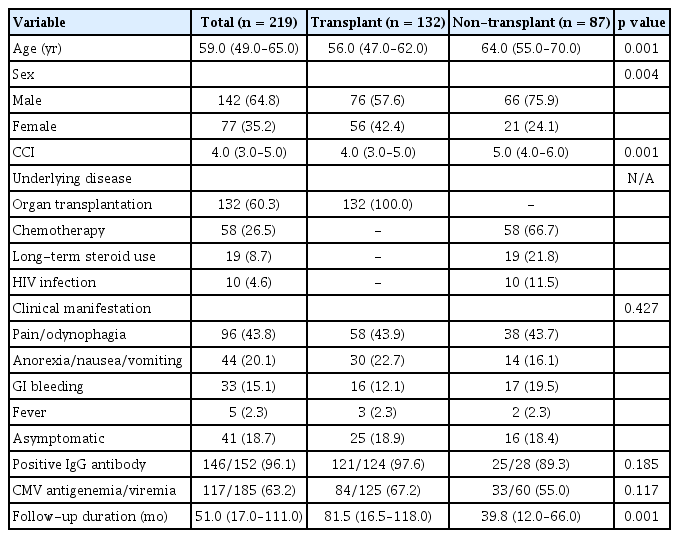
Table 1 presents the baseline characteristics of the 219 eligible patients. Among these patients, 132 (60.3%) and 87 (39.7%) were transplant and non-transplant patients, respectively. The median age of the patients was 56.0 and 64.0 years in the transplant and non-transplant groups, respectively (p = 0.001). In addition, the median CCI score was significantly higher in the non-transplant group than in the transplant group (5.0 vs. 4.0, p = 0.001). The most common symptoms were pain and odynophagia among patients in the transplant and non-transplant groups (43.9% vs. 43.7%). Among the 152 patients who underwent serum CMV immunoglobulin G (IgG) antibody test, 146 (96.1%) showed positivity for serum IgG antibodies against CMV. In addition, among the 185 patients who underwent serum CMV antigenemia/viremia test, 117 (63.2%) exhibited CMV antigenemia or viremia. The median follow-up period was 51.0 months (IQR, 17.0–111.0 mo).
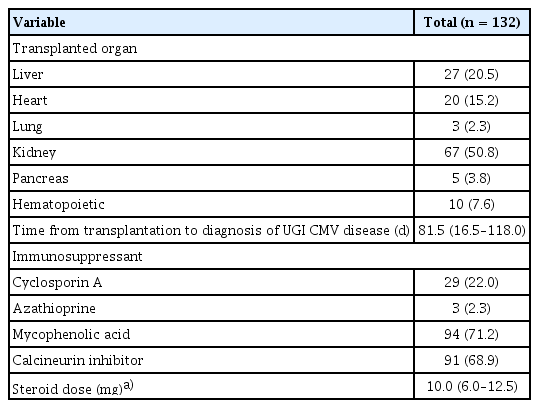
Table 2 summarizes the clinical characteristics of the transplant group. Kidney transplantation (50.8%) was the most common type of surgery, followed by liver (20.5%) and heart (15.2%) transplantations. The median time from transplantation to the diagnosis of UGI CMV disease was 81.5 days (IQR, 16.5–118.0 d). Mycophenolic acid and calcineurin inhibitors were administered to 94 (71.2%) and 91 (68.9%) patients, respectively. All patients also received a low-dose steroid to maintain immunosuppression, with a median dose of 10.0 mg (IQR, 6.0–12.5 mg).
Endoscopic characteristics
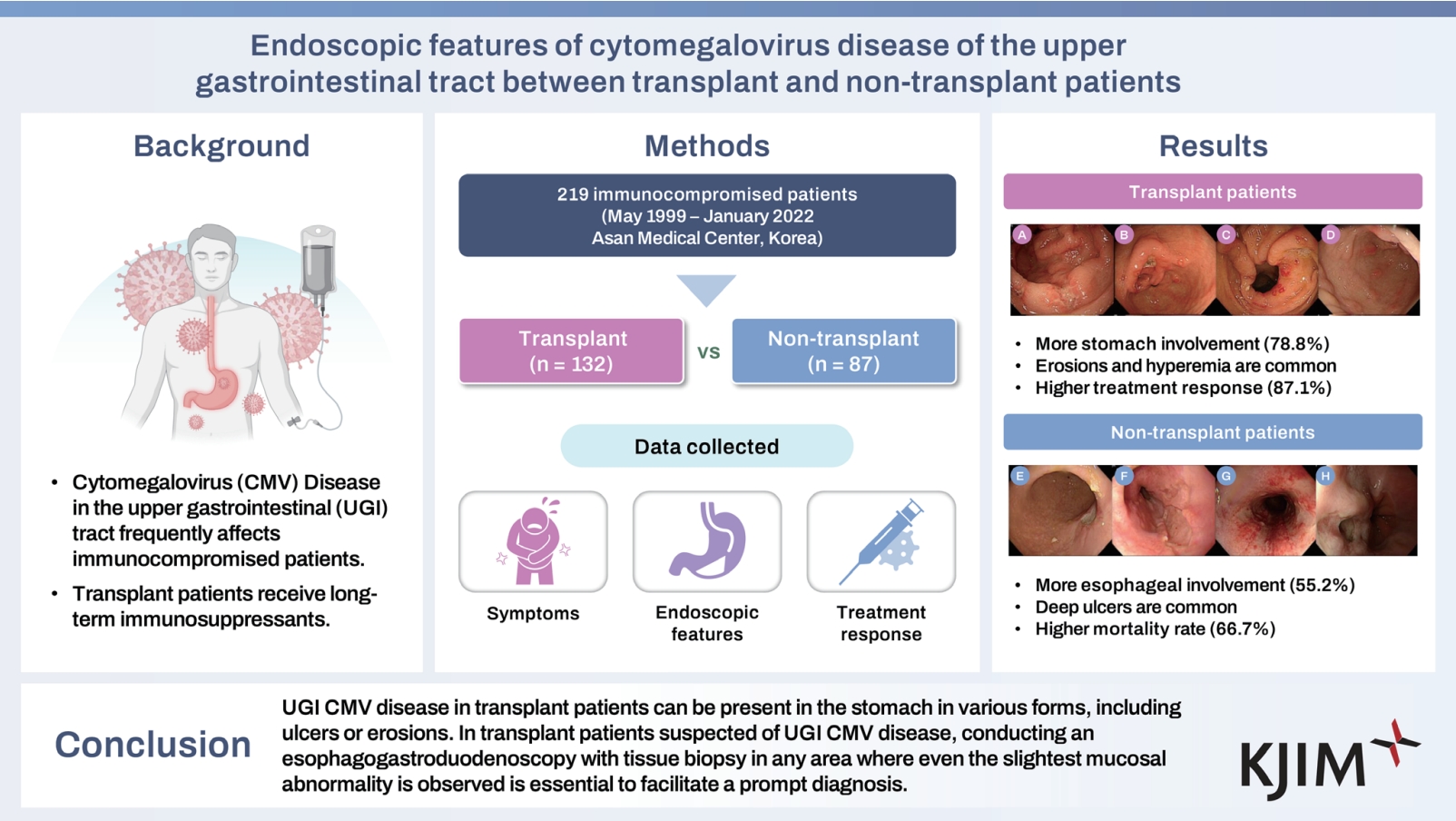
Table 3 presents the endoscopic features of the patients with UGI CMV disease. The stomach was the most commonly affected site in the transplant group (with an incidence of 78.8% in the transplant group vs. 46.0% in the non-transplant group; p < 0.001). In contrast, the esophagus was the most commonly affected site in the non-transplant group (with an incidence of 24.2% in the transplant group and 55.2% in the non-transplant group; p < 0.001).
Ulcers were more frequently found among the patients in the non-transplant group than among those in the transplant group (92.0% vs. 57.6%, p < 0.001), with most of the ulcers being active-stage ulcers (59.8% vs. 31.8%). However, mucosal erosion and hyperemia were more commonly observed among the patients in the transplant group than among those in the non-transplant group (42.4% vs. 5.7%, p < 0.001). Representative EGD images from the transplant and non-transplant groups are presented in Figure 2.
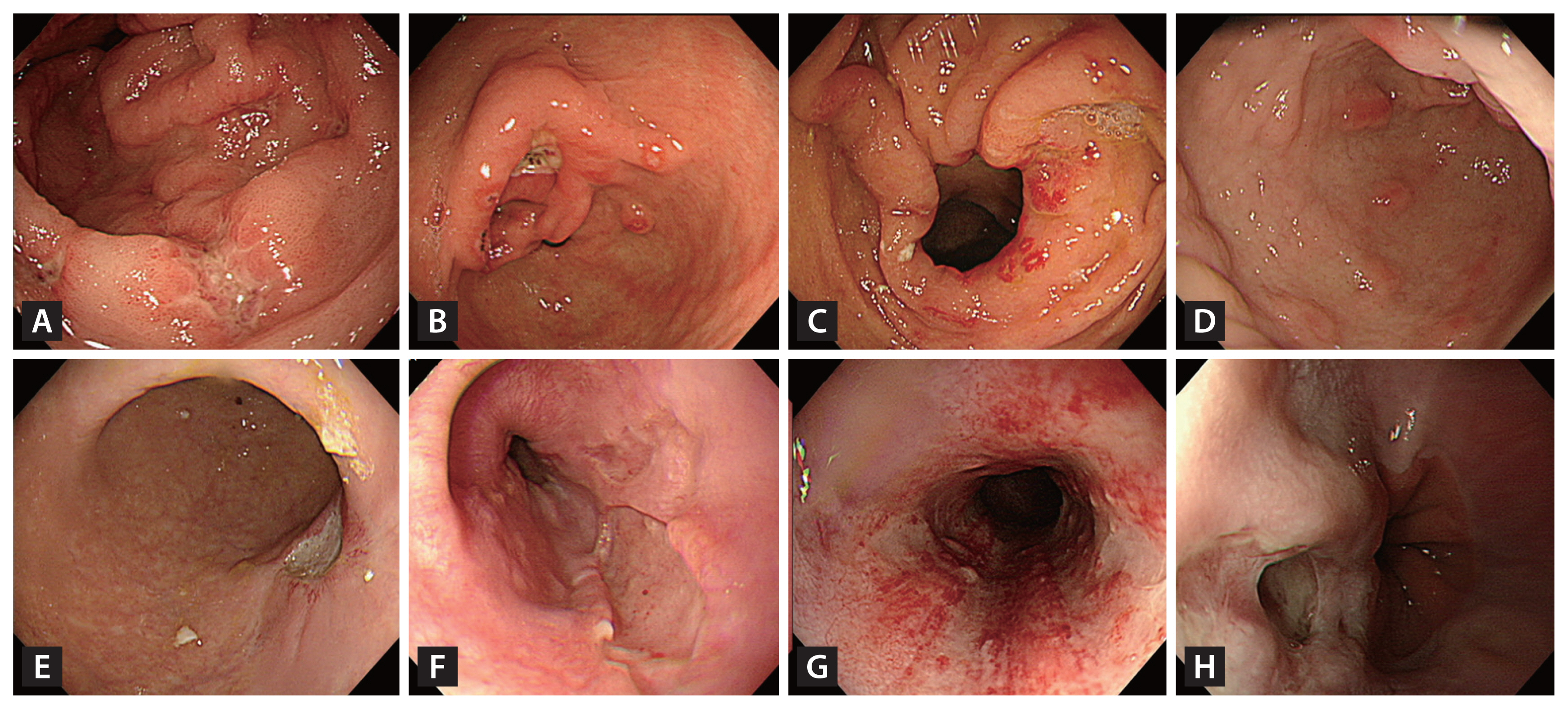
Endoscopic images of UGI CMV disease. (A) through (H) are endoscopic images from patients diagnosed with UGI CMV disease. Among these, (A) to (D) are from transplant patients, while (E) to (H) are from non-transplant patients. (A) Multiple erosions and a healing-stage ulcer in the antrum. (B) A deep, healing-stage ulcer with multiple erosions and linear superficial hyperemia in the antrum. (C) Multiple erosions and hyperemia around the pyloric ring. (D) Multiple raised erosions in the antrum. (E) A deep, healing-stage ulcer at the gastric angle. (F) A deep, geographic ulcer in the lower esophagus. (G) Multiple geographic ulcers with mucosal hyperemia in the mid-esophagus (H) A deep ulcer with surface exudate in the lower esophagus. UGI, upper gastrointestinal; CMV, cytomegalovirus.
Overall, 20 patients (9.1%) exhibited multiorgan CMV disease. In the transplant group, 11 patients (5.0%) were diagnosed with multiorgan CMV disease, including eight cases (3.7%) of CMV enterocolitis, two cases (0.9%) of CMV pneumonia, and one case (0.5%) of CMV retinitis. In the non-transplant group, nine patients (4.1%) were diagnosed with multiorgan CMV disease, including four cases (1.8%) of CMV enterocolitis and five cases (2.3%) of CMV retinitis.
Clinical outcomes
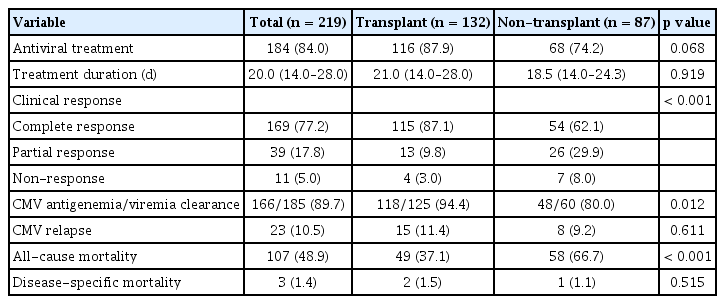
Table 4 presents the clinical outcomes of patients with UGI CMV disease. Among the patients in the transplant group, 116 (87.9%) underwent antiviral treatment, with drugs such as ganciclovir, valganciclovir, and foscarnet, with a median treatment duration of 21.0 days. However, 68 (74.2%) patients received antiviral treatment in the non-transplant group, with a median treatment duration of 18.5 days. The complete response rate (87.1% vs. 62.1%, p < 0.001), as well as CMV antigenemia/viremia clearance rate (94.4% vs. 80.0%, p = 0.012), was significantly higher in the transplant group than in the non-transplant group. Three patients (1.4%) showed delayed clinical improvement after ganciclovir administration and were subsequently treated with foscarnet. Among them, two showed improvement with foscarnet treatment. All three patients had undergone bone marrow transplantation for hematologic diseases. None of the transplant patients experienced graft dysfunction due to UGI CMV disease. Twenty-three (10.5%) patients experienced CMV relapse, among whom eight (3.7%) had an endoscopic relapse of CMV disease. The all-cause mortality rate was significantly higher in the non-transplant group than in the transplant group (66.7% vs. 37.1%, p < 0.001). However, disease-specific mortality showed no significant differences between the transplant and non-transplant groups (1.5% vs. 1.1%, p = 0.515). Figure 3 shows the overall endoscopic findings and treatment results of the patients.

Summary of clinical findings and treatment results of patients with UGI CMV disease. When CMV involved two or more organs, it was classified as ‘multiple UGI,’ while involvement of a single organ was labeled as ‘esophagus,’ ‘stomach,’ or ‘duodenum,’ depending on the affected organ. UGI, upper gastrointestinal; CMV, cytomegalovirus.
DISCUSSION
Our study aimed to examine the clinical characteristics of UGI CMV disease in immunocompromised patients, particularly in patients who underwent transplantation. We found that transplant patients experience UGI CMV disease at a relatively young age compared with non-transplant patients. The disease primarily manifests in the stomach and typically presents as erosions rather than ulcers alone. In contrast, among non-transplant patients, CMV infection occurred more frequently in older male patients with greater comorbidities and mainly presented as esophageal ulcers.
The strength of this study lies in the long-term follow-up (median: 51 mo) period and a large sample size of 219 patients with UGI CMV disease. To the best of our knowledge, this is the largest study analyzing patients with UGI CMV disease. Previous studies on UGI CMV disease were mostly published in the form of case series [11], had an insufficient number of patients [12,13], or had relatively short median follow-up periods [14], which were perceived as limitations of those studies. In this study, we analyzed medical records and endoscopy data collected over approximately 23 years, including a larger number of patients with UGI CMV disease than those included in the previous studies. Additionally, we included patients who were followed up for a longer period compared with those in the previous studies.
In this study, 43.8% of patients reported symptoms, including chest or abdominal pain and odynophagia, as well as other symptoms, such as nausea, vomiting, and anorexia. These findings align with the results of previous studies on UGI CMV disease [5,15,16]. However, 18.7% of patients did not report distinct GI symptoms; they remained asymptomatic despite the presence of distinct mucosal lesions detected during routine endoscopic surveillance. This observation is consistent with the findings of previous studies, which reported that 7–17% of patients were asymptomatic and incidentally diagnosed with GI CMV disease during their EGD or computed tomography examination [16,17]. Given the diverse clinical presentations of GI CMV disease, considering the possibility of UGI CMV disease and performing appropriate histological diagnosis in immunocompromised patients, even if they are asymptomatic when minor mucosal lesions are detected on EGD, is essential.
Diagnosing UGI CMV disease can be challenging due to its varying symptoms, locations, and appearances [16,18,19]. In particular, its similar appearance and location to other viral infections, such as herpes simplex virus infection, can lead to diagnostic difficulties. According to previous studies, esophageal involvement is the most common presentation of UGI CMV disease and is typically characterized by a deep ulcer with clear margins [1,5,17]. However, contrary to previous research findings, our study revealed a higher incidence of erosions in the stomach among transplant patients, while ulcers were more frequently detected in the esophagus among non-transplant patients. Furthermore, clinical responses indicated a higher rate of complete response in the transplant group than in the non-transplant group, likely due to the milder manifestation of UGI CMV disease in this group. These differences underscore the variations in clinical presentations depending on the underlying disease of the patient. The cause of these differences can be inferred through several hypotheses. First, in the case of transplant patients, UGI CMV disease may have been relatively milder due to the administration of CMV prophylaxis for several days immediately after transplantation surgery. In addition, GI involvement of CMV may be relative to the patient’s underlying condition rather than due solely to their immunosuppression state. In fact, the non-transplant patients included in this study were older than the transplant patients and had a significantly higher baseline CCI due to their underlying diseases, such as cancer, connective tissue disorder, and HIV infection. Therefore, even if the endoscopic finding is not the typical deep ulcer with a clear margin commonly observed when a patient with GI symptoms is in an immunosuppressed state, such as transplantation, chemotherapy, long-term steroid use, or HIV infection, conducting a careful observation and thorough biopsy is crucial. Additional histopathological examinations, such as IHC or PCR, are also essential to establish a definitive diagnosis of GI CMV disease.
In this study, tissue IHC and PCR were found to be positive in 97.3% and 88.6% of patients with UGI CMV disease, respectively. However, CMV antigenemia/viremia was detected in only 63.2% of tested patients. Most CMV infections occur in the latent stage following primary infection and usually lead to reactivation as T-cell immunity declines [3]. In transplant patients, CMV infection can occur when a CMV seronegative recipient receives an organ from a CMV seropositive donor [20,21]. However, according to a study investigating CMV seroprevalence in Koreans from 1995 to 2015, CMV seropositivity was confirmed in 94.1% of the study population [22]. Considering this, the incidence of new CMV infections through transplanted organs is believed to be significantly low. In contrast, CMV seropositivity in this study was confirmed in 96.1% of patients at the time of UGI CMV disease diagnosis, and while CMV detection in the bloodstream was relatively low, more cases of CMV were detected in tissue. This suggests that not only systemic CMV reactivation but also local CMV reactivation in target organs contribute to UGI CMV disease. Therefore, GI CMV disease cannot be completely ruled out, even in patients with symptoms when no CMV antigenemia or viremia occurred.
According to previous studies, GI CMV disease in transplant patients is known to lead not only to infectious diseases, such as bacteremia and invasive fungal disease, but also to graft dysfunction, including acute or chronic rejection, ultimately impacting patients’ mortality and morbidity [2,23–27]. However, in the analysis results of this study, the transplant and non-transplant groups showed significantly low disease-specific mortality rates of 1.5% and 1.1%, respectively. In addition, no cases of graft dysfunction due to CMV disease occurred in the transplant group. This could be attributed to the nature of UGI CMV disease, which typically presents with easily recognizable symptoms upon onset, and the accessibility of EGD in Korea, allowing for early diagnosis and treatment through EGD examination. While the all-cause mortality rate was significantly higher in the non-transplant group than in the transplant group, this can be attributed to the presence of patients with advanced cancer who underwent chemotherapy, resulting in a higher mortality rate due to their disease progression.
This study had some limitations. First, this was a single-center retrospective study, and the number of patients with UGI CMV disease was relatively small. Second, some patients were diagnosed with UGI CMV disease but did not receive antiviral treatment, which could have influenced the response evaluation. Third, baseline characteristics, including age, sex, and CCI, differed between the transplant and non-transplant groups. Consequently, these differences may have influenced the study results. Nevertheless, these variations are considered valuable for informing the treatment of patients with UGI CMV disease in real-world scenarios, as they provide insights into CMV infection in actual clinical settings. Lastly, this study excluded immunocompetent patients due to an insufficient number of cases, which made comparative analysis challenging. The absence of a comparison between immunocompetent and immunocompromised patients may have limited the study results. Although a study has investigated GI CMV disease in immunocompetent patients, most of the GI CMV disease cases primarily involved the colon, with relatively rare instances of UGI involvement [12]. Therefore, if conducted in the future with a larger patient population, including those with immunocompetent patients, the study may provide valuable insights into the specific characteristics of UGI CMV disease in immunosuppressed patients.
In conclusion, compared to typical UGI CMV disease, UGI CMV disease in transplant patients usually manifests in the stomach and can present in various forms, including ulcers or erosions. Therefore, when a transplant patient reports UGI symptoms, conducting an EGD and performing a tissue biopsy in any area where even the slightest mucosal abnormality is observed is essential to facilitate a prompt diagnosis.
KEY MESSAGE
1. Among the immunocompromised patients undergoing transplant, UGI CMV disease primarily affects relatively younger patients and presents with milder symptoms and erosions in the stomach.
2. A significant portion of patients experienced chest or abdominal pain, but 18.7% were asymptomatic despite mucosal lesions detected during routine endoscopy, highlighting the importance of thorough biopsy and histological diagnosis even in the absence of symptoms.
Notes
CRedit authorship contributions
Yuri Kim: conceptualization, methodology, investigation, data curation, formal analysis, writing - original draft; Do Hoon Kim: conceptualization, methodology, data curation, formal analysis, writing - original draft; Myeongsook Seo: data curation; Hee Kyong Na: data curation; Kee Wook Jung: data curation; Ji Yong Ahn: data curation; Jeong Hoon Lee: data curation; Kee Don Choi: data curation; Ho June Song: data curation; Gin Hyug Lee: data curation; Hwoon-Yong Jung: data curationss
Conflicts of interest
The authors disclose no conflicts.
Funding
None