Clinical approach to sexually transmitted infections and pelvic inflammatory disease in women with acute pyelonephritis
Article information
Abstract
Background/Aims
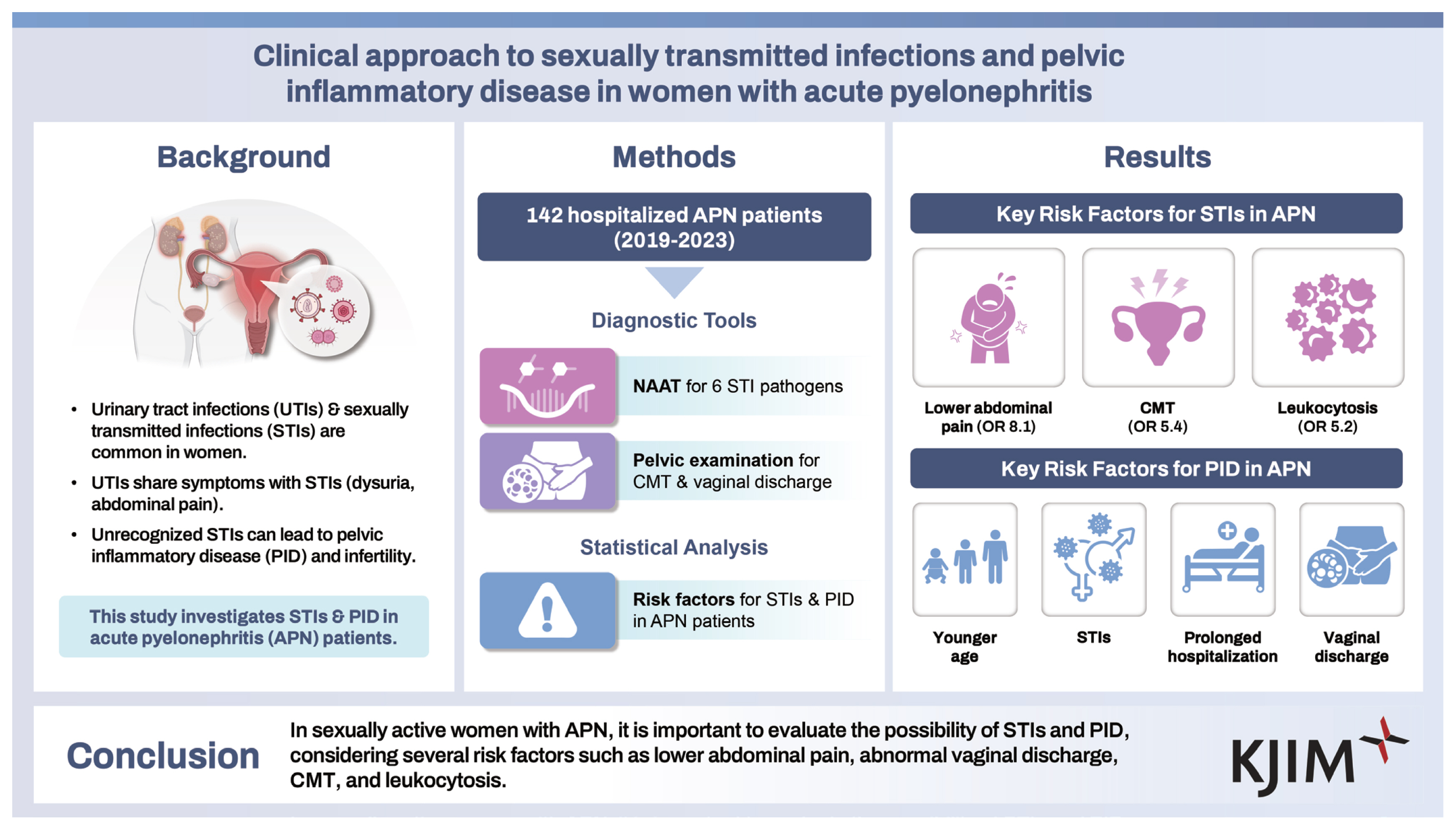
This study aimed to investigate co-occurrence and clinical characteristics of sexually transmitted infections (STIs) and pelvic inflammatory disease (PID) in women hospitalized for acute pyelonephritis (APN).
Methods
This single-center retrospective study reviewed medical records of inpatients with APN from January 2019 to February 2023 and identified records of 142 patients who were referred to a gynecologist to evaluate gynecological diseases including STIs.
Results
Of the 142 patients, 47 were tested positive for sexually transmitted pathogens in nucleic acid amplification testing, confirming the presence of STIs. In patients with APN, those with STIs were more likely to have lower abdominal pain or cervical motion tenderness (CMT) on pelvic examination and leukocytosis (> 14.5 × 109/L) than those without STIs. Of the 93 patients who underwent pelvic examination, 34 had CMT with one or more of additional criteria for the clinical diagnosis of PID, such as abnormal vaginal discharge and leukorrhea confirmed by microscopic examination, which could be clinically diagnosed as PID.
Conclusions
In sexually active women with APN, it is important to evaluate the possibility of STIs and PID, considering several risk factors such as lower abdominal pain, abnormal vaginal discharge, CMT, and leukocytosis.
INTRODUCTION
Urinary tract infections (UTIs) are common bacterial infections that are more prevalent in women than in men. UTIs frequently coexist with sexually transmitted infections (STIs) in sexually active patients [1–3]. Notably, previous studies [3–7] have found that 10–50% of women with UTIs have STIs. UTIs and STIs share similar risk factors (such as sexual intercourse) with similar symptoms, including dysuria and lower abdominal pain. Therefore, it is challenging to distinguish STIs from UTIs. STIs are often ignored or underdiagnosed. Thus, they continue to spread in an undertreated state. The Centers for Disease Control and Prevention (CDC) estimated that approximately 26 million new cases of STIs occurred in the United States in 2018, with a total of 68 million infections. The economic burden of STIs is considerable. Annual direct medical costs of new infections alone totaled approximately $16 billion in the United States [8,9]. STIs can lead to severe clinical sequelae, including pelvic inflammatory disease (PID), infertility, cervical cancer, and chronic pelvic pain [7].
Therefore, early diagnosis and treatment of STIs are important and a more proactive approach to screening and evaluation is necessary in patients with UTIs.
PID is a syndrome characterized by inflammatory processes, including endometritis, salpingitis, tubo-ovarian abscess, and pelvic peritonitis. PID can be caused by vaginal flora, respiratory or enteric pathogens, and STIs, leading to infertility, ectopic pregnancy, and chronic pelvic pain, similar to effects of STIs. Symptoms and signs of PID, such as fever and abdominal pain, are similar to those of upper UTIs, such as acute pyelonephritis (APN). However, investigations on the association between APN and PID are limited [10–12].
Thus, this study aimed to investigate co-occurrence and clinical characteristics of STIs and PID in women hospitalized for APN.
METHODS
Study design
This retrospective study included patients hospitalized for nonobstructive APN at St. Vincent’s Hospital of the Catholic University in Suwon, Korea, from January 2019 to February 2023. The study protocol was approved by the Institutional Review Board (IRB) of St. Vincent’s Hospital of the Catholic University (IRB reference number: VC23RISI0205). This study was conducted in accordance with the tenets of the Declaration of Helsinki. The IRB waived the requirement for written informed consent because of its retrospective design.
Patient population and definitions
Women aged at least 18 years with the potential for sexual activity who were admitted to St. Vincent’s Hospital with non-obstructive APN were included in this study. Criteria for diagnosing APN were: fever ≥ 38.5°C or the presence of fever or chills 24 hours before admission and the presence of at least one of the following: (1) flank pain; (2) costovertebral angle tenderness on physical examination; (3) at least one new or worsening symptom of lower UTI (dysuria, urgency, frequency, sense of residual urine, and suprapubic pain); and (4) pyuria (≥ 10 white blood cells [WBCs] per high-power field [hpf]) [13,14]. STI was diagnosed with nucleic acid amplification tests (NAATs) for six vaginal pathogens: Chlamydia trachomatis, Mycoplasma genitalium, Mycoplasma hominis, Ureaplasma urealyticum, Trichomonas vaginalis, and Neisseria gonorrhoeae. PID diagnosis required at least one of the minimum clinical criteria of CDC, including cervical motion tenderness (CMT), uterine tenderness, and adnexal tenderness on pelvic examination. Additional criteria to increase the specificity of diagnosis included the following: (1) tympanic temperature ≥ 38.3°C; (2) abnormal cervical mucopurulent discharge or cervical friability; (3) leukorrhea confirmed by microscopic examination (≥ 10 WBC/hpf in vaginal fluid); (4) elevated erythrocyte sedimentation rate; (5) elevated C-reactive protein level; and (6) laboratory documentation of cervical infection with N. gonorrhoeae or C. trachomatis [12,15]. Therefore, PID was diagnosed with CMT on pelvic examination and at least one of the additional criteria in this study.
Data collection
We retrospectively collected data on baseline demographic characteristics, underlying diseases, and clinical signs and symptoms, including urinary and genital tract symptoms, physical examination findings, history of UTI or PID, laboratory findings, microbiological data, and time to defervescence. Results of abdominal computed tomography (CT) scans were collected for all patients. Vaginal swabs were obtained during gynecological consultations for saline microscopy, NAATs, and culture.
Statistical methods
Categorical variables were summarized using frequencies and percentages. Continuous variables were summarized using the Mann–Whitney U-test. Chi-square test or Fisher’s exact test was performed to compare categorical data between groups. Logistic regression was used to assess risk factors for STIs. In addition, multivariable logistic regression analysis was performed to identify independent risk factors for STIs and PID in patients with APN. Statistical significance was set at p < 0.05. R version 4.2.2 (R Core Team, 2022) was used for all statistical analyses.
RESULTS
Clinical characteristics
A total of 841 patients hospitalized for APN between January 2019 and February 2023 were identified. Patients were referred for gynecological consultation for various reasons, including complaints of vaginal discomfort, such as vaginal discharge and itching, detection of gynecological abnormalities on abdominal CT, and issues related to menstruation or menopause. A total of 142 patients underwent evaluations by a gynecologist for possibility of STIs and PID. The gynecologist performed a pelvic examination and a vaginal swab test, including Gram staining, culture, and NAATs, to evaluate genital tract infections. Abdominal CT scan results for 142 patients revealed no cause of fever other than UTI. HIV and syphilis screenings were negative for all participants. Among 142 patients, 47 (33.1%) had an STI identified using NAATs, which could detect six vaginal pathogens: C. trachomatis, M. genitalium, M. hominis, U. urealyticum, T. vaginalis, and N. gonorrhoeae.
Comparison of clinical features and laboratory results between STI-positive and -negative groups
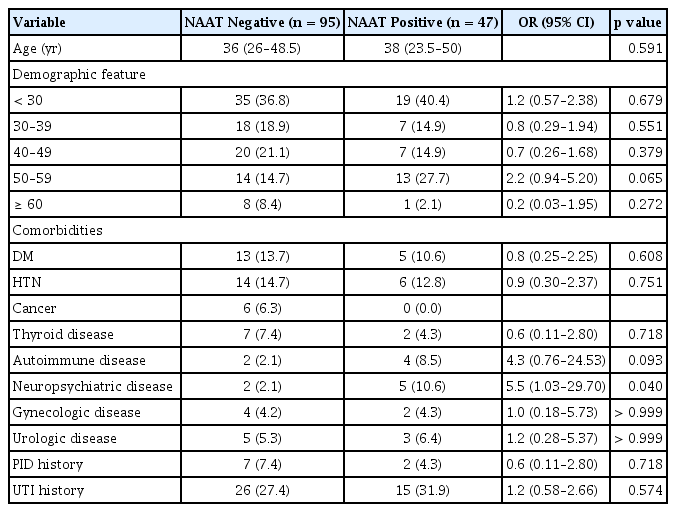
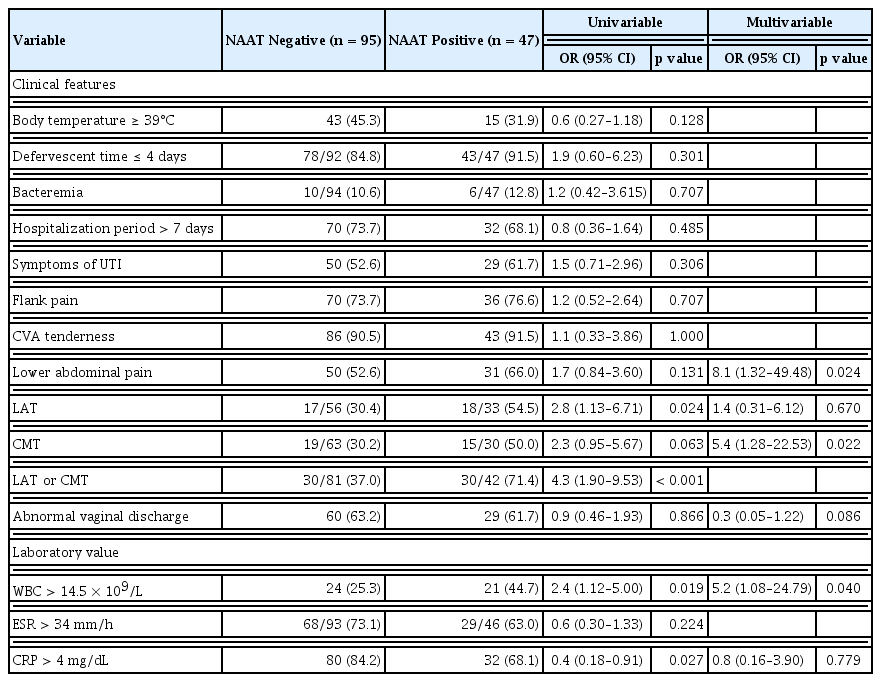
In the comparison between STI-positive and STI-negative groups, the proportion of neuropsychiatric diseases was significantly higher in the STI-positive group (10.6% [5/47] vs. 2.1% [2/95]; odds ratio [OR], 5.5; p = 0.040) (Table 1). In the medical record review, records of lower abdominal tenderness and CMT were available for 89 and 93 patients, respectively. The number of patients with either lower abdominal tenderness or CMT was significantly higher in the STI-positive group than in the STI-negative group (71.4% [30/42] vs. 37.0% [30/81]; OR, 4.3; p < 0.001). Multiple logistic regression analysis showed that lower abdominal pain, CMT, and leukocytosis (> 14.5 × 109/L) were associated with positive NAAT results for sexually transmitted pathogens. Lower abdominal pain was identified as the most significant risk factor for STIs (OR, 8.1; 95% confidence interval [CI], 1.32–49.48) (Table 2).
Clinical significance of CMT in APN patients
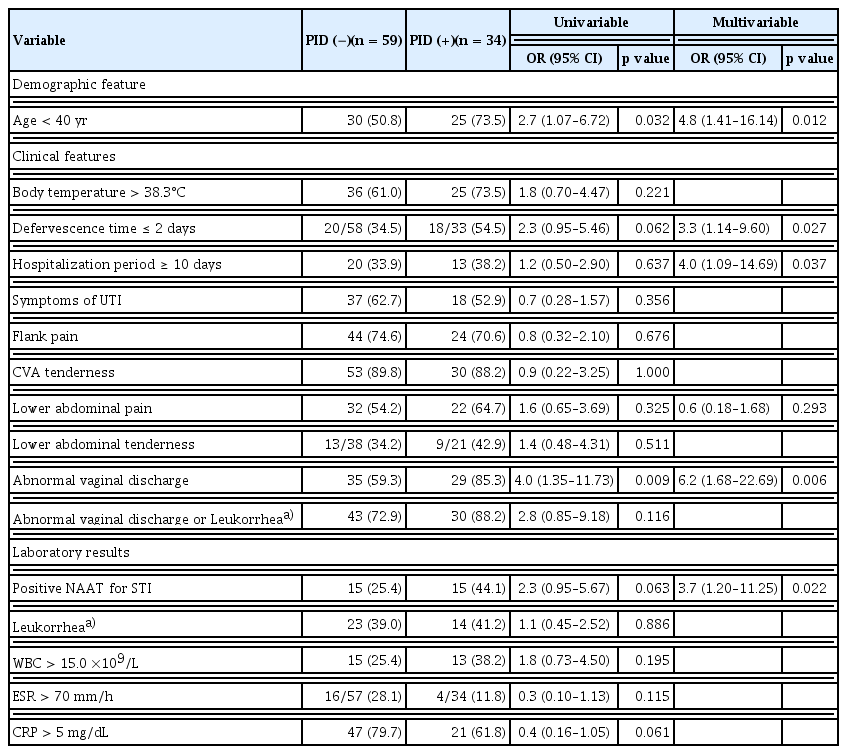
Patients with APN were divided into two groups based on the presence or absence of CMT on pelvic examination. PID could be clinically diagnosed in patients with CMT and one or more additional criteria according to clinical criteria of the CDC [15]. All 34 patients with CMT in this study met one or more of the additional criteria for clinical diagnosis of PID [12,15]. Therefore, among the 93 patients who underwent pelvic examinations in this study, all 34 patients exhibiting CMT were clinically diagnosed with PID (34/93, 36.6%). Most (30/34, 88.2%) of these 34 patients clinically diagnosed with PID exhibited one or both of the important components of the additional criteria: the presence of abnormal vaginal discharge and leukorrhea confirmed by microscopic examination (WBC ≥ 10/hpf in vaginal fluid). Among the 34 patients clinically diagnosed with PID, the proportion of patients with STIs was 44.1% (15/34), which was higher than that in patients without PID (25.4% [15/59]; OR, 2.3, p = 0.063). Multivariable logistic regression analysis showed that the presence of STIs indicated by positive NAAT results and abnormal vaginal discharge was significantly associated with PID. Moreover, a short defervescence time (≤ 2 days), long hospitalization period (≥ 10 days), and age < 40 years were risk factors for PID (Table 3).
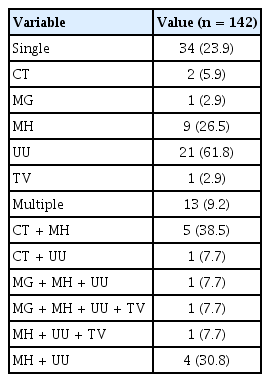
Distribution of six sexually transmitted pathogens in the STI-positive group of patients
Among the six sexually transmitted pathogens, U. urealyticum was the most prevalent (20%, 29/142), followed by M. hominis (15%, 21/142), C. trachomatis (6%, 8/142), M. genitalium (2%, 3/142), and T. vaginalis (2%, 3/142). N. gonorrhoeae was not detected in the present study. The proportion of patients with co-infections involving two or more pathogens was 27.7% (13/47) (Table 4).
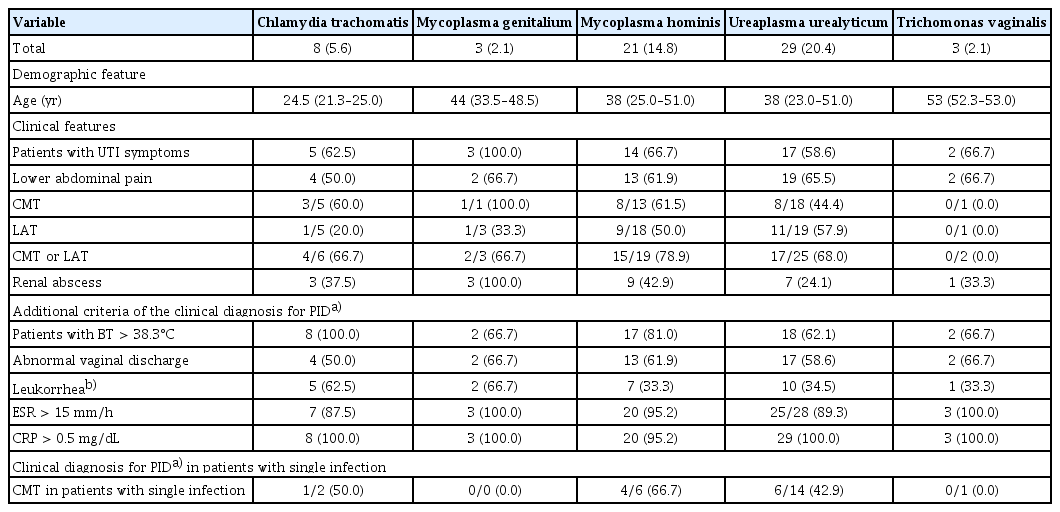
Comparison of clinical characteristics and outcomes by patient group with specific sexually transmitted pathogen
Table 5 shows baseline demographics, clinical characteristics, and laboratory results for patients infected with specific sexually transmitted pathogens. Of patients with five sexually transmitted pathogens, all patients with CMT met one or more of the additional criteria for clinical diagnosis of PID. Proportions of patients clinically diagnosed with PID were 50.0% (1/2), 0.0% (0/0), 66.7% (4/6), 42.9% (6/14), and 0.0% (0/1) for C. trachomatis, M. genitalium, M. hominis, U. urealyticum, and T. vaginalis, respectively (Table 5).
DISCUSSION
Previous studies have investigated coinfection with STIs in women with UTI, with reported prevalence of coinfection in the range of 10% to 50% [3,5,6,16]. In this study, 33.1% (47/142) of patients with APN had concurrent STIs. APN with STIs was independently associated with lower abdominal pain, leukocytosis, and CMT. Of these three risk factors, lower abdominal pain was most strongly associated with STIs. Therefore, it is essential to inquire about the presence of lower abdominal pain in patients with APN to screen for STIs.
PID, a severe complication of STIs, shares overlapping clinical features with APN including fever and abdominal pain. This is attributable to ascending infections originating in the lower urinary and genital tract. However, investigations on the association between APN and PID are limited.
The prevalence of PID has been shown to be 10–14% among women of reproductive-age [17]. In the present study, the prevalence of PID, defined by the presence of CMT was estimated to be 36.6% (34/93), which was approximately twice as high as those reported in previous studies of patients without APN. In this study, a significant majority (85.3%, 29/34) of patients with APN with clinically diagnosed PID reported abnormal vaginal discharge. This proportion was notably higher than the 56% reported in a study by Mitchell and Prabhu [12], which focused on patients with endometritis and salpingitis. Furthermore, the prevalence of leukorrhea in patients with APN who were clinically diagnosed with PID was also higher in this study than that in the study by Mitchell and Prabhu [12] (41% vs. 22%). Such a high prevalence of symptoms and signs related to the genital tract observed in this study suggests that the prevalence of PID is high in APN patients.
In this study, the presence of STIs was significantly associated with PID. The OR determined by multivariable logistic regression analysis was 3.7. Previous studies have reported that N. gonorrhoeae and C. trachomatis are primary causes of PID. However, N. gonorrhoeae was not detected in the present study and C. trachomatis was present in only 6% of PID cases. Recent studies have reported a declining trend in the prevalence of N. gonorrhoeae. Non-chlamydial and non-gonococcal causes of PID have been reported to account for 9–23% of cases. Normal vaginal flora, including facultative anaerobes and enteric gram-negative rods, bacterial vaginosis-associated pathogens, and microorganisms such as M. genitalium, M. hominis, U. urealyticum, and T. vaginalis, can also cause PID [10,15]. Therefore, the presence of STIs such as M. genitalium, M. hominis, U. urealyticum, and T. vaginalis in this study appears to have a significant relationship with PID. The lower urinary tract and vagina can share urogenital microbiome. Microorganisms associated with PID encompass not only STIs, but also enteric bacteria. However, it remains unclear whether these latter entities are causative agents of PID or they were merely from an represent opportunistic growth owing to alterations in the microbiome of the upper genital tract. Nevertheless, recent studies have considered these important PID markers [11,18]. In this study, the higher prevalence of PID than the prevalence of STIs among patients with APN could be related to the microbiome in the urogenital tract.
In NAAT results of this study, the most common pathogen was U. urealyticum, which could colonize the genital tract. It is also considered an opportunistic pathogen that can cause gynecological diseases such as preterm delivery, infertility, and spontaneous abortion [19–22]. The colonization rate of U. urealyticum in the vagina ranges from 7% to 42% [23]. In this study, the proportion of U. urealyticum was 20% and the prevalence of PID in patients with APN infected with Ureaplasma urealyticum was found to be high at 44.4% (8/18). Previous studies did not provide clear evidence that U. urealyticum could cause PID. However, a previous study by Lee et al. [24] has reported that the most frequently identified pathogen is U. ureaplasma in patients with PID or Fitz-Hugh-Curtis Syndrome, accounting for 63.6% (42/66) of cases. In the present study, the prevalence of PID in patients with APN with a single U. ureaplasma infection was also high (42.9%). These results suggest that U. ureaplasma might be an important marker for PID. Therefore, if U. ureaplasma is detected in the vaginal fluid of sexually active patients with APN, the possibility of PID should be evaluated.
C. trachomatis is one of the most common STI pathogens worldwide. It can cause endometritis, PID, ectopic pregnancy, and infertility. A study conducted by Rowley et al. [25] has estimated that the global prevalence of C. trachomatis infection in women ranges from 1.5% to 7.0%, similar to the prevalence of 5.6% (8/142) found in the present study. Furthermore, 3 (60.0%) of 5 APN patients with C. trachomatis infection were clinically diagnosed with PID, presenting with CMT. In this study, the median age of patients with C. trachomatis infection was lower than that of patients infected with other pathogens. Therefore, young women with APN should be evaluated for C. trachomatis infection and PID, considering associated complications such as ectopic pregnancy and infertility.
This study has several limitations due to its retrospective design and characteristics of STIs. First, sexually transmitted pathogens identified in this study were detected in vaginal fluid samples rather than in samples collected from the upper genital tract. Therefore, the causative agent of PID could not be confirmed. However, PID is one of the severe complications of STI. A previous study has reported that the prevalence of PID among women with STIs is 94% [10]. Therefore, it is likely that STIs identified in this study are closely related to the etiology of PID. The presence of PID without detectable STIs in this study might warrant consideration of their relationships with other undetected pathogens. Further research on this aspect is necessary. Second, PID diagnosis was based on the minimum clinical criteria recommended by the CDC. Although this approach has a high sensitivity to include as many people at risk of PID as possible, it has the limitation of a low specificity. To histologically or microbiologically confirm PID, invasive procedures are necessary. However, they can lead to delays in the diagnosis and treatment of PID, increasing the burden of the disease [10]. Consequently, PID diagnosis usually relies on CDC clinical criteria. However, further histological and microbiological studies are required to better understand the pathogenesis of PID in patients with APN. Third, the 142 participants in this study were suspected of having an STI or PID. They subsequently underwent a pelvic examination or vaginal swab test by gynecologists. This could introduce a selection bias. The high prevalence of STIs or PID observed in this study might be associated with this bias. Further research is needed to more accurately determine the prevalence of STIs or PID in patients with APN.
In conclusion, it is essential to screen for concurrent STIs or PID based on clinical findings, such as abdominal pain or vaginal symptoms, when managing APN in women with potential sexual activity.
KEY MESSAGE
1. It is necessary to screen for concurrent STIs or PID for patients with APN.
2. Lower abdominal pain in patients with APN was most strongly associated with STIs.
3. U. urealyticum was the most common NAAT result in this study. It might be an important marker for PID.
Notes
CRedit authorship contributions
Mi-Hee Kim: conceptualization, methodology, investigation, data curation, formal analysis, validation, writing - original draft, writing - review & editing, visualization; Hyojin Ahn: methodology, investigation, data curation; Soyeon Kang: methodology, investigation, data curation; Ahra Lee: methodology, investigation, data curation; Seong-Heon Wie: conceptualization, methodology, validation, writing - review & editing, supervision, funding acquisition
Conflicts of interest
The authors disclose no conflicts.
Funding
We thank the Research Institute of Medical Science at St. Vincent’s Hospital for providing financial support (SVHR-2020-06).