Clinical benefits and future directions of medium cut-off membranes in hemodialysis: a comprehensive review
Article information
Abstract
Medium cut-off (MCO) membranes have emerged as a promising innovation in hemodialysis (HD), offering enhanced clearance of large middle-molecules of uremic toxins compared to traditional HD membranes, while maintaining minimal loss of albumin. The introduction of MCO membranes represents a significant advancement in dialysis technology, potentially reducing the risk of complications associated with inadequate removal of toxins. Compared to high-flux membranes, MCO membranes demonstrate superior efficacy in eliminating large middle-molecules without excessive loss of beneficial proteins, such as albumin. The clinical benefits of MCO membranes extend beyond toxin clearance. They improve quality of life, reduce erythropoiesis-stimulating agent doses and resistance, lower hospitalization rates, and decrease overall healthcare costs. Currently, there is insufficient evidence regarding the effects of MCO membranes on cardiovascular diseases and mortality. Further studies are required to assess their effects on patient outcomes and long-term survival. Future innovations in membrane technology, coupled with ongoing research and development, have the potential to enhance dialysis efficacy further, reduce complications, and facilitate the development of eco-friendly solutions. Additional studies are required to fully explore the potential of MCO membranes and refine their clinical application.
INTRODUCTION
As kidney function declines and progresses to end-stage kidney disease (ESKD), kidneys lose their ability to remove waste products effectively. Consequently, patients with ESKD require kidney replacement therapy such as dialysis or kidney transplantation. Hemodialysis (HD) involves the movement of blood and dialysates across the HD membrane to ensure efficient dialysis. Water and solutes pass through semipermeable membranes via distinct separation mechanisms such as diffusion and ultrafiltration. These mechanisms rely on the pressure gradient created by the patient’s blood to allow water and solutes to move toward the dialysate side. HD is critical for removing waste products and regulating the fluid balance, particularly for clearing uremic toxins. For decades, continuous efforts have been made to optimize uremic toxin removal using HD membranes. Standard high-flux dialysis membranes are not highly effective at removing middle-molecular-weight uremic toxins, particularly those larger than 15 kDa. Although online hemodiafiltration (HDF) utilizing high-flux membranes combines convection and diffusion to broaden the range of uremic toxins, it also presents challenges, such as the need for high vascular access flow, ultrapure water, and large convective volumes. Recent advancements in dialysis membranes aim to enhance convective clearance while limiting albumin loss to improve the elimination of middle-molecular-weight toxins [1]. Consistent with these advancements, a medium cut-off (MCO) membrane, a next-generation dialysis membrane, was developed to enable expanded HD by effectively removing the middle molecules. This review aims to provide a comprehensive overview of MCO membranes and their clinical implications, discussing their advantages over traditional dialysis membranes and their potential to improve patient outcomes in ESKD.
EVOLUTION OF HD MEMBRANES AND UREMIC TOXIN CLASSIFICATION
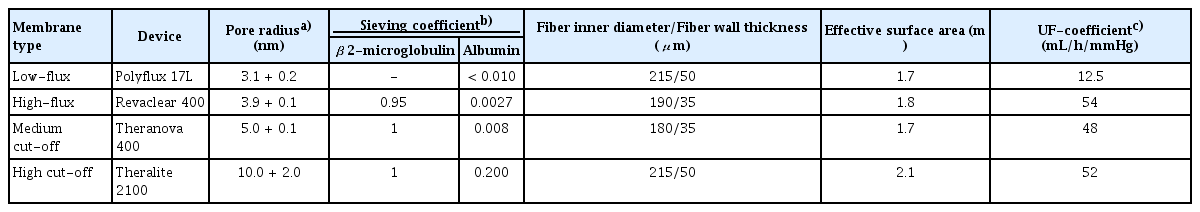
HD membrane development is a continuous process. HD membranes are classified into cellulosic and synthetic types, based on their composition [2]. Recently, synthetic membranes have become predominant, and a classification method based on the ultrafiltration coefficient has been proposed. HD membranes were initially classified as having low or high permeability based on differences in water membrane permeability. Subsequent research further refined this classification into low- and high-flux membranes, based on their ability to remove fluids and molecules [3]. Low- and high-flux membranes are categorized according to their ultrafiltration coefficients (Kuf), which represent the pore sizes of the membranes. High-flux dialyzers are defined as having a Kuf > 20 mL/h/mmHg, whereas low-flux dialyzers are defined as having a Kuf < 10 mL/h/mmHg. Furthermore, acknowledging the critical role of albumin in human health, researchers have revised the classification to incorporate factors such as water permeability, beta-2 microglobulin clearance, and albumin-related parameters [4].
As kidney function declines, uremic toxins accumulate and are traditionally classified as small water-soluble molecules, protein-bound solutes, or middle molecules [5]. When not used in HDF mode, high-flux dialysis membranes can remove uremic toxins with a molecular weight of approximately 15 kDa [6]. Accordingly, uremic toxins > 15 kDa are classified as large middle-molecules, with further subdivisions based on the molecular weight and clearance capacity of the dialysis membranes. Recently, experts have suggested refining the classification of middle molecular uremic toxins, emphasizing the need to consider their molecular structure, removal methods, and correlation with clinical symptoms, which calls for an update of the current framework [7]. Uremic toxicity has harmful effects on various organs and metabolic processes. Previous studies have demonstrated that uremic toxins with molecular weights greater than 15 kDa, such as cytokines, adipokines, and hormones, contribute to chronic inflammation, atherosclerosis, structural heart disorders, and secondary immunodeficiency [6]. Therefore, it is important to clarify the relationship between the accumulation of specific toxins, their diverse physicochemical characteristics, and their clinical symptoms. A more detailed classification of uremic toxins has been proposed based on the removal capabilities of the currently available dialysis membranes [7]. Molecules are categorized as: small (< 0.5 kDa), small-middle (0.5–15 kDa), medium-middle (> 15–25 kDa), large-middle (> 25–58 kDa), and large (> 58–170 kDa). High-flux dialyzers used in standard HD can clear molecules up to 15 kDa, with this threshold increasing to 25 kDa when used in HDF. A new class of membranes, the MCO membrane, allows the removal of molecules of up to 56 kDa. Compared to high-flux membranes, MCO membranes demonstrate superior clearance efficiency for larger molecules within the 25–56 kDa range.
EMERGENCE AND OPERATIONAL PRINCIPLES OF MCO MEMBRANES
The MCO membrane, a new-generation dialysis membrane, was developed to enable expanded HD by effectively removing middle molecules up to 56 kDa. The characteristics and classification of the HD membranes are summarized in Table 1. In comparison, HCO membranes developed earlier to eliminate free light chains (kappa and lambda) in patients with myeloma kidney are associated with significant albumin loss owing to their large pore size, which limits their use for short-term therapeutic purposes [8].
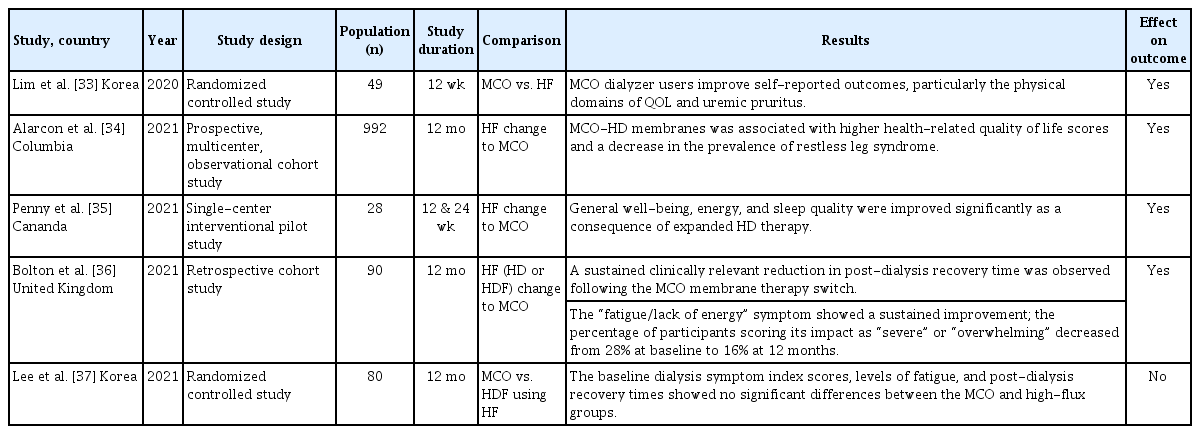
An MCO membrane was developed to enable the clearance of large middle-molecules while minimizing albumin loss. The MCO membrane was designed with tailored pore sizes and optimized to accommodate different molecular sizes while achieving an effective pore size (Stokes–Einstein radius) [9]. The Stokes–Einstein radius of the molecular weight cutoff was aligned with the effective pore radius of the membrane. MCO membranes feature an improved pore size distribution and adopt a tighter configuration to enable effective removal of large middle-molecules in clinical practice while minimizing albumin loss [6,10]. Moreover, the MCO membrane increases internal filtration by reducing the inner diameter and wall thickness compared to traditional high-flux membranes, thereby enhancing its convective flow capacity (Fig. 1) [9]. This results in greater permeability owing to significant internal filtration in the proximal section, driven by an increased end-to-end pressure drop [11]. The improved convective transport along the fibers facilitates the removal of larger molecules with low diffusion coefficients. Adequate backfiltration in the distal part of the MCO membranes eliminates the need for exogenous substitution fluids [12]. These unique sieving and filtration properties enable MCO membranes to provide expanded clearance of uremic toxins in the 15–45 kDa range, offering a performance comparable to that of high-volume HDF and superior to that of standard high-flux HD membranes. However, some earlier studies have reported that the removal efficiency of middle molecules, such as myoglobin and kappa- and lambda-free light chains, was superior in case of HDF compared with MCO membranes [13,14]. Other studies have demonstrated superior reduction ratios for MCO membranes [15]. Notably, the convective volumes used in these HDF studies varied considerably—30.4 ± 4.1 L per session in the study by Maduell et al. [13] and 23.5 ± 3.8 L in the study by Reque et al. [15]—suggesting that higher convective volumes may enhance solute clearance. Consequently, HDF requires specific conditions to achieve optimal performance, including high convective volumes, high vascular access flow rates, and consistent use of ultrapure dialysate. These technical requirements may limit their broad application in clinical settings. In contrast, MCO membranes, which can be used with a conventional dialysis infrastructure, may offer a more practical and accessible alternative for improving middle-molecule clearance in routine clinical practice. However, evidence on the long-term clinical impact of MCO membranes, including their effects on cardiovascular events and both cardiovascular and all-cause mortality, remains limited, highlighting the need for further research.
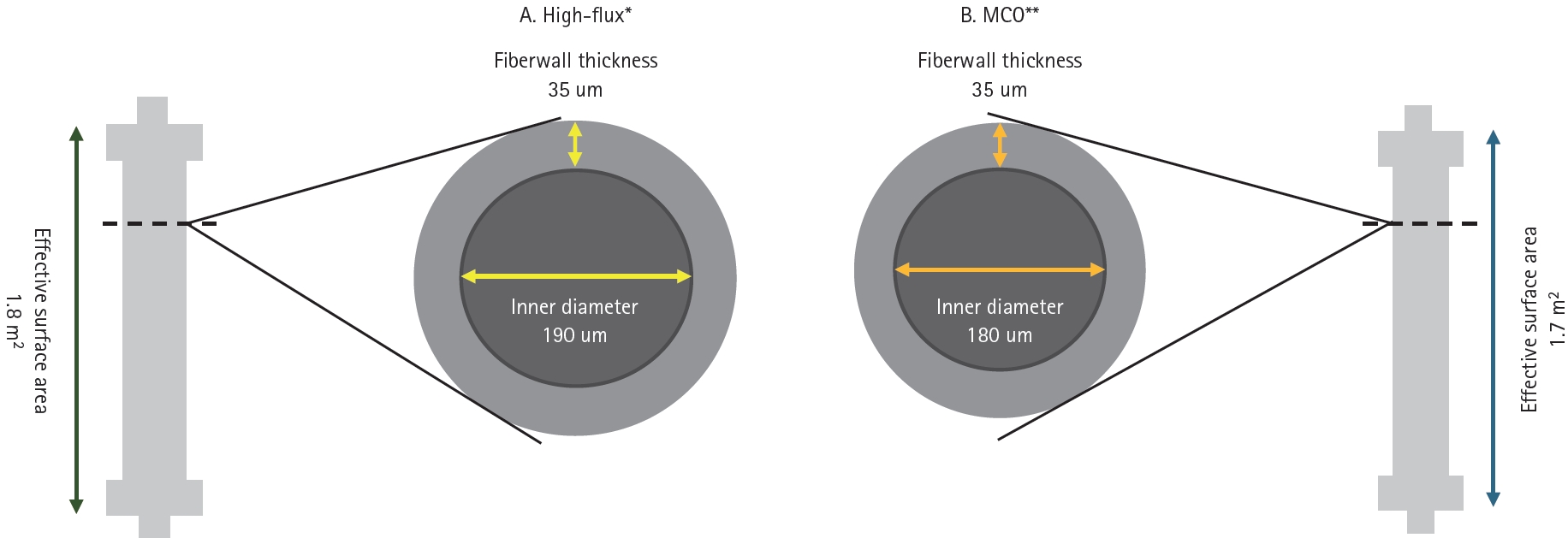
Comparison of fiber wall thickness and inner diameter in the high-flux and MCO membranes. *Revaclear 400, **Theranova 400. MCO, medium cut-off.
There have been concerns that using an MCO membrane for HD could result in higher albumin loss than with high-flux HD and HDF [16]. In a 12-month study of 638 patients undergoing extended HD with an MCO membrane, serum albumin levels showed minimal reduction, with a nadir of 3.9 g/dL [17]. Variations remained within 5% of the baseline, and mean levels remained within the normal range (3.5–5.5 g/dL). Previous studies have shown that serum albumin loss is comparable between MCO membrane use, high-flux HD, and HDF [18-20]. Over three years of treatment with MCO membranes, an evaluation of clinical safety revealed no significant differences in serum albumin levels over time compared to the HD group (mean monthly change difference = -0.0003, p = 0.855) [21]. Additionally, albumin loss with the use of an MCO membrane was lower than that observed with peritoneal dialysis [22]. Thus, although the use of MCO membranes may lead to a slight decrease in serum albumin levels, the reduction was not significant, and the levels generally remained within the normal range, indicating that they can be safely used.
CLINICAL BENEFITS OF THE MCO MEMBRANE
Enhanced removal of large middle-molecules of uremic toxins
The MCO membrane demonstrates superior removal of large middle-molecular uremic toxins. Previous studies have shown that using an MCO membrane results in better clearance of large middle-molecule uremic toxins, such as myoglobin (17.8 kDa), kappa-free light chains (25 kDa), and lambda-free light chains (50 kDa) than using a high-flux membrane [18,23]. A meta-analysis including multiple randomized and nonrandomized controlled trials showed that the use of MCO membranes significantly reduced the levels of β2-microglobulin, kappa-free light chains, and lambda-free light chains compared to high-flux membranes [24-26]. Furthermore, an additional in vitro study demonstrated that incubating a human vascular endothelial cell line with dialyzed serum resulted in significantly lower levels of nuclear factor-κB and Bax in the MCO membrane group than in the high-flux membrane group [18]. These findings suggest that the MCO membrane plays a potentially beneficial role in the apoptosis of the human endothelium. Large middle-molecule uremic toxins in patients with chronic kidney disease (CKD) are significant contributors to atherosclerosis, inflammation, vascular calcification, and cardiovascular diseases [6,27-30]. Therefore, as an advanced new-generation dialyzer, the MCO membrane is expected to offer several advantages, particularly the ability to remove large and middle-sized molecules. In our previous study, the MCO membrane demonstrated significantly higher removal efficiency for large middle-molecules related to vascular calcification, such as FGF23, OPG, and sclerostin, than a high-flux membrane [31]. In another study, after 12 months of using the MCO dialyzer, plasma sclerostin levels did not significantly increase compared to those in the control group, and the reduction ratios of substances such as sclerostin, FGF23, and retinol-binding protein 4 were enhanced [32]. Additional long-term studies are needed to assess the clinical outcomes of patients on HD following the removal of these large middle molecules.
Uremic symptom and quality of life (QOL) improvement in patients with HD
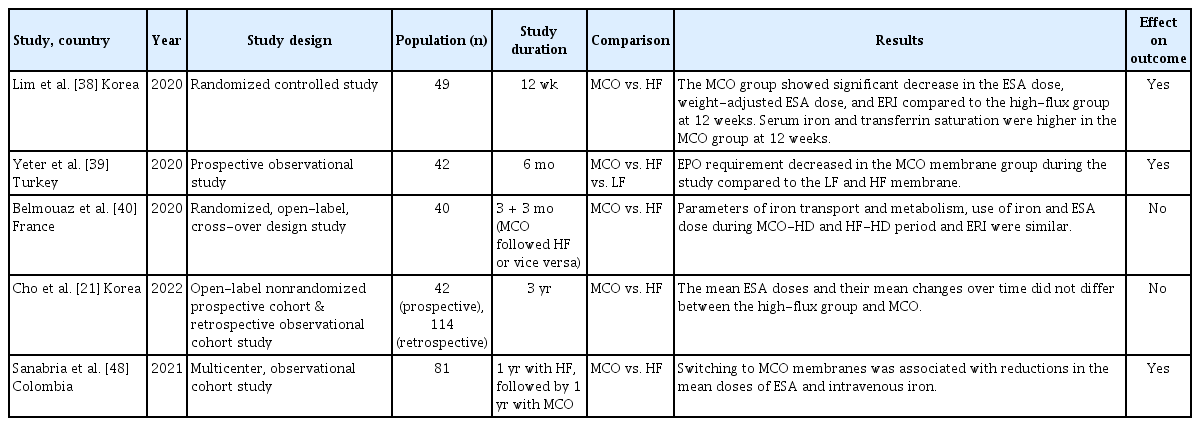
In patients undergoing HD, the accumulation of large middle-molecule uremic toxins contributes to issues such as reduced QOL, restless leg syndrome, and uremic pruritus. Previous studies have demonstrated improved QOL outcomes with the use of MCO membranes (Table 2). A randomized controlled study compared MCO membranes with high-flux membranes over 12 weeks in patients undergoing maintenance HD [33]. QOL was evaluated at baseline and after 12 weeks using the Kidney Disease Quality of Life Short Form-36, whereas pruritus was assessed using a questionnaire and visual analog scale. The results demonstrated that the MCO group experienced significant improvements in physical functioning and the physical role components of QOL. Additionally, there was a reduction in the distribution of morning pruritus and scratching frequency during sleep. A study evaluating patient-reported outcomes 12 months after changing from high-flux to MCO membranes found that MCO-HD was linked to improved health-related QOL scores and a reduced prevalence of restless legs syndrome (22.1% at baseline to 10% at 12 months) [34]. Another study reported significant improvements in general well-being, energy levels, and sleep quality after transitioning to an MCO membrane [35]. Another study, which used surveys conducted every three months over a year following the switch to MCO membranes, revealed a reduction in postdialysis recovery time and improved perceived fatigue levels [36]. However, some studies have not observed improvements in QOL with the use of MCO membranes. In a 12-month follow-up randomized controlled study, there were no significant differences between the MCO and high-flux groups in baseline dialysis symptom index scores, fatigue levels, or recovery times after dialysis [37]. Further long-term studies with a broader patient population are required to determine which individuals would benefit the most from MCO membranes.
Improvement in erythropoiesis stimulating agent resistance
Uremic toxins and chronic inflammation can disrupt iron metabolism and impair the response to erythropoiesis-stimulating agents (ESA) in patients undergoing dialysis. Given these effects, the use of MCO membranes has shown benefits in ESA management (Table 3). A 12-week randomized controlled trial comparing MCO membranes with high-flux membranes in maintenance HD patients revealed that the MCO group experienced a significant reduction in the ESA dose, weight-adjusted ESA dose, and erythropoietin resistance index (ERI) [38]. Moreover, the serum iron and transferrin saturation levels were notably higher in the MCO group after 12 weeks. Another study examining changes over 6 months with MCO, high-flux, and low-flux membranes found that ESA requirements decreased in the MCO membrane group compared to the high- and low-flux membrane groups [39]. However, some studies did not find any significant effects. In a crossover study in which patients underwent MCO for 3 months, followed by high-flux for 3 months (or vice versa), the parameters related to iron transport and metabolism, iron usage, ESA dose, and ERI were similar between the MCO-HD and high-flux HD periods [40]. Throughout the 3-year study period, no significant differences were observed in the average doses of darbepoetin (ESA) or their changes over time between the high flux and MCO groups [21]. Based on these studies, although the precise mechanism has not been demonstrated, expanded HD using an MCO membrane may benefit erythropoiesis, overcome ESA resistance, and not seem to harm HD patients.
Effect of the MCO membrane on cardiovascular parameters and cardiovascular and all-cause mortality
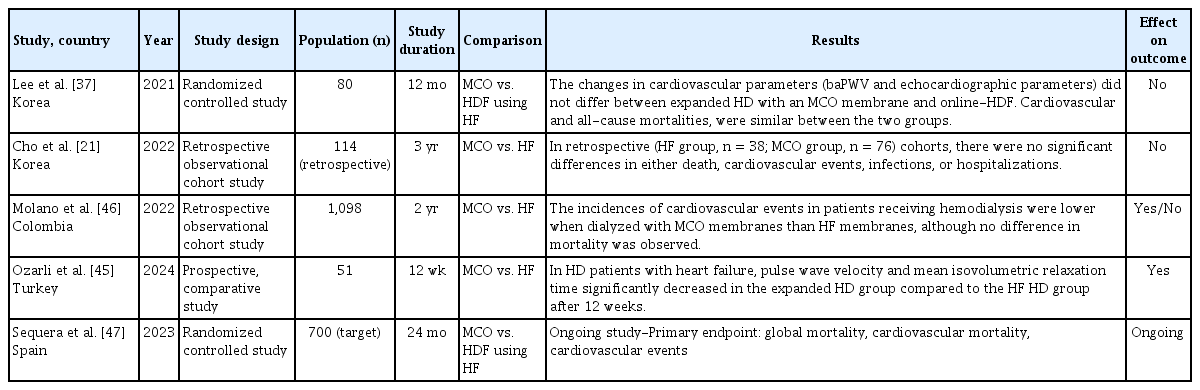
The ultimate goal of effectively removing uremic toxins is to reduce morbidity and mortality in HD patients, particularly by improving cardiovascular disease, which is the leading cause of death in patients undergoing HD [41-44]. Consequently, research has continued to explore the impact of MCO membranes on cardiovascular disease, cardiovascular mortality, and all-cause mortality (Table 4). In a 1-year randomized controlled trial comparing expanded HD using MCO membranes and online HDF, there were no significant differences in brachial-ankle pulse wave velocity or echocardiographic parameters between the two groups [37]. Coronary artery calcium scores remained stable in the online HDF group, whereas an increasing trend was observed in the expanded-HD group. The expanded HD and online HDF groups exhibited similar risks of cardiovascular and all-cause mortalities. In a 3-year observational study, there were no significant differences between the groups that used MCO membranes and the high-flux group in terms of mortality, cardiovascular events, infections, or hospitalizations [21]. A small-scale, short-term, single-center, prospective, parallel-group comparative study showed that in HD patients with heart failure, the group using MCO membranes achieved superior removal of uremic toxins and inflammatory biomarkers compared to the high-flux HD group [45]. Additionally, the pulse wave velocity and mean isovolumetric relaxation time significantly decreased after 12 weeks. In a retrospective, observational, multicenter cohort study involving 1,098 HD patients followed up for two years, the frequency of nonfatal cardiovascular events was significantly lower in the MCO group than in the high-flux HD group [46]. However, no significant differences in all-cause mortality rates were observed. Currently, a multicenter, open-label, prospective, randomized study is being conducted in Spain: the MOTheR HDx study trial (NCT03714386) [47]. This trial aimed to compare expanded HD using MCO membranes with online HDF by enrolling 350 patients in each group with a planned follow-up period of 24 months. The composite primary endpoint was to determine whether expanded HD was not inferior to online HDF in terms of global mortality, cardiovascular mortality, and cardiovascular events. Secondary outcomes included changes in hospitalization rates, QOL, ESA responsiveness, and other related factors. This study is currently underway, and the results will provide important guidance for the management of patients undergoing HD. Future research should focus on well-powered randomized controlled trials with a long-term follow-up to elucidate the potential benefits of MCO membranes on cardiovascular outcomes and mortality. These studies should incorporate robust cardiovascular endpoints such as major adverse cardiovascular events, cardiac biomarker trends, and echocardiographic parameters. Moreover, subgroup analyses based on comorbid conditions (e.g., diabetes, established cardiovascular disease, or high inflammatory status) may help identify populations that would benefit the most from MCO therapy. Head-to-head comparisons with established modalities such as high-volume HDF are also crucial for delineating the relative clinical value of MCO membranes.
Other clinical benefits of the MCO membrane
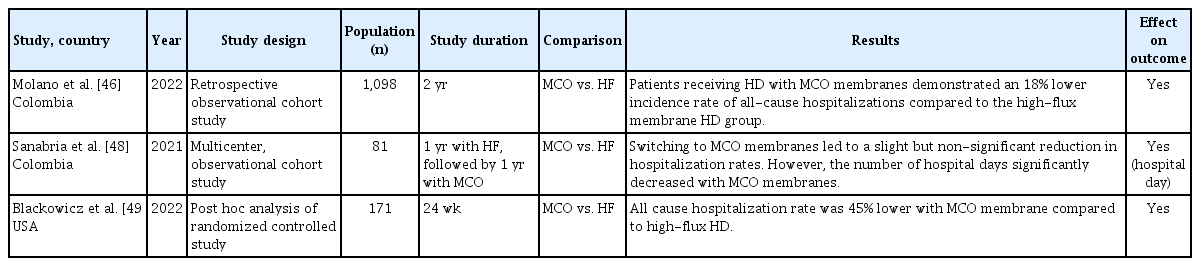
Other clinical benefits of MCO membranes have been reported in studies of patients undergoing HD. Several studies have evaluated the effects of MCO membranes on hospitalization rates (Table 5). In a retrospective study with a 2-year follow-up, patients receiving HD with MCO membranes demonstrated an 18% lower incidence rate of all-cause hospitalization than those receiving high-flux membrane HD [46]. After 1 year of using high-flux membranes, switching to MCO membranes for another year resulted in a minor, nonsignificant reduction in hospitalization rates (high-flux vs. MCO: 0.77 vs. 0.71 events/patient-year, p = 0.698) [48]. However, the number of hospital days significantly decreased with MCO membranes (high-flux vs. MCO: 5.94 vs. 4.41 days, p = 0.0001). In another study, a post hoc analysis of a 24-week randomized controlled trial found that MCO membranes were associated with a 45% lower all-cause hospitalization rate than high-flux membranes [49]. However, the length of hospital stay was similar between the two groups.
Metabolomics and proteomics, alongside transcriptomics and genomics, represent key areas within the “omics” research spectrum [50]. They offer insights into the genome’s “functional” outcomes and are widely applied in kidney disease research [51]. In our previous study, high-flux and MCO membranes were used consecutively for five weeks each, followed by metabolomic and proteomic analyses [52]. During the period of MCO membrane usage, our findings indicated that the use of MCO dialyzers led to distinct metabolomic and proteomic profiles compared to high-flux dialyzers. These profiles may be associated with oxidative stress, inflammation, insulin resistance, the complement–coagulation axis, and nutritional factors.
An in vitro study showed that cell-free hemoglobin (CFH) produced by mechanically stressed erythrocytes during extracorporeal therapies was reduced by MCO membranes during HD [53]. Building on these findings, a clinical study was conducted to investigate the effects of 12 months of MCO membrane use [19]. The results showed that CFH concentrations significantly decreased during the HD sessions in the MCO group. However, no significant changes in serum CFH concentrations were observed from baseline to 12 months in either the MCO or high-flux groups.
In a recent randomized controlled study, the MCO group showed a significantly smaller decline in glomerular filtration rate over 12 months than the high-flux group. It maintained a higher 24-h urine volume for up to 9 months [54]. The MCO membrane facilitates the removal of a broad range of middle-molecular-weight uremic toxins and inflammatory mediators such as kappa- and lambda-free light chains, TNF-α, and GDF-15, which may contribute to the preservation of residual renal function. Although the mechanisms underlying these renoprotective effects require further investigation, our findings suggest that MCO membranes offer meaningful clinical benefits to patients undergoing HD.
ECO-FRIENDLY DIALYSIS MEMBRANE
HD is a life-saving therapy; however, it can have environmental impacts as it consumes considerable amounts of water and energy, and generates significant waste. Additionally, the amount of water used for HD varies depending on the treatment and modality applied. In this context, MCO membranes offer eco-friendly properties. HDF consumes substantially more water and dialysis fluid than conventional or expanded HD [55]. This results in increased power consumption and waste production. Moreover, HDF requires the use of a second sterilizing ultrafilter, which further increases the filter usage and contributes to additional waste production. MCO membranes offer benefits comparable to HDF but require less water because of their filtration–backfiltration capabilities [56]. Additionally, the use of an MCO membrane can reduce both power consumption and waste production compared to HDF. Furthermore, MCO membranes are smaller and lighter than high-flux membranes [56], which helps reduce the weight of medical waste. Beyond the clinical effects of MCO membranes, these environmental impacts align with the goals of green dialysis [57,58].
POTENTIAL CHALLENGES AND LIMITATIONS
Most studies using MCO membranes for HD have reported no additional adverse events or serious complications associated with the procedure. Although MCO dialyzers show promising results in removing middle-molecule uremic toxins, there are limited long-term data on their impact on patient outcomes such as survival rates and cardiovascular health. In a Korean three-year cohort study comparing MCO membranes with high-flux membranes, there were no significant differences in clinical efficacy and safety outcomes during the treatment period [21]. The levels of inflammatory cytokines remained stable, and there were no differences in the rates of death, cardiovascular events, infections, or hospitalization between the groups. Additional clinical trials should consider the diverse characteristics of patients undergoing dialysis in order to determine the long-term safety and potential benefits of MCO membranes.
MCO membranes are not effective in removing gut-derived protein-bound uremic toxins (PBUTs) and large-molecule uremic toxins [20,59]. PBUTs such as indoxyl sulfate and p-cresyl sulfate are associated with cardiovascular disease in patients with CKD [60]. Previous studies have shown that post-dialysis plasma levels of indoxyl sulfate and p-cresyl sulfate are not significantly different among high-flux HD, HDF, and MCO-HD [20].
MCO dialyzers tend to be more expensive than conventional high-flux membranes, hindering their widespread adoption, particularly in resource-limited settings. However, the reduction in hospitalization rates and shorter hospital stays associated with MCO membrane use could lower overall healthcare costs, making it important to consider these potential savings in the evaluation [48,49]. For instance, one study reported that the use of MCO membranes was significantly associated with fewer hospitalization days and reduced doses of medications, including ESA, iron supplements, antihypertensive drugs, and insulin [61]. This study estimated a 23% reduction in the annual hospitalization-related costs in the MCO group. Another study suggested that the use of an MCO membrane could lead to a 45% reduction in hospitalization rates and approximately USD 6,098 in annual healthcare cost savings per patient [49]. Future research, particularly large-scale randomized controlled trials, are required to confirm and strengthen the findings of this study. Further cost-effectiveness analyses are warranted to comprehensively evaluate the economic implications of adopting MCO membranes in various health care settings.
CONCLUSION
MCO membranes represent a significant advancement in HD, offering improved removal of large middle-molecules of uremic toxins compared to traditional HD membranes while maintaining minimal loss of albumin. MCO membranes have been shown to improve patient QOL, reduce ESA dose and resistance, lower hospitalization rates, and decrease overall healthcare costs. However, important clinical questions remain unresolved, including the long-term safety of MCO membranes, their cost-effectiveness across diverse healthcare settings, and their impact on adverse outcomes, such as cardiovascular events and all-cause mortality. It is also important to identify the patient population that may benefit the most from MCO membrane therapy, as its effects could vary depending on individual patient characteristics. Future research should address these gaps through large-scale, multicenter randomized controlled trials and real-world data analyses. As nephrology continues to evolve toward patient-centered and precision-based care, the role of MCO membranes should be further explored as part of a broader strategy to improve dialysis quality and long-term patient outcomes.
Notes
CRedit authorship contributions
Hyo Jin Kim: conceptualization, methodology, investigation, writing - original draft, writing - review & editing; Sang Heon Song: conceptualization, methodology, investigation, writing - review & editing, supervision
Conflicts of interest
The authors disclose no conflicts.
Funding
None