Predicted pro-inflammatory high-sensitivity C-reactive protein score and inflammatory bowel disease: a cross-sectional study
Article information
Abstract
Background/Aims
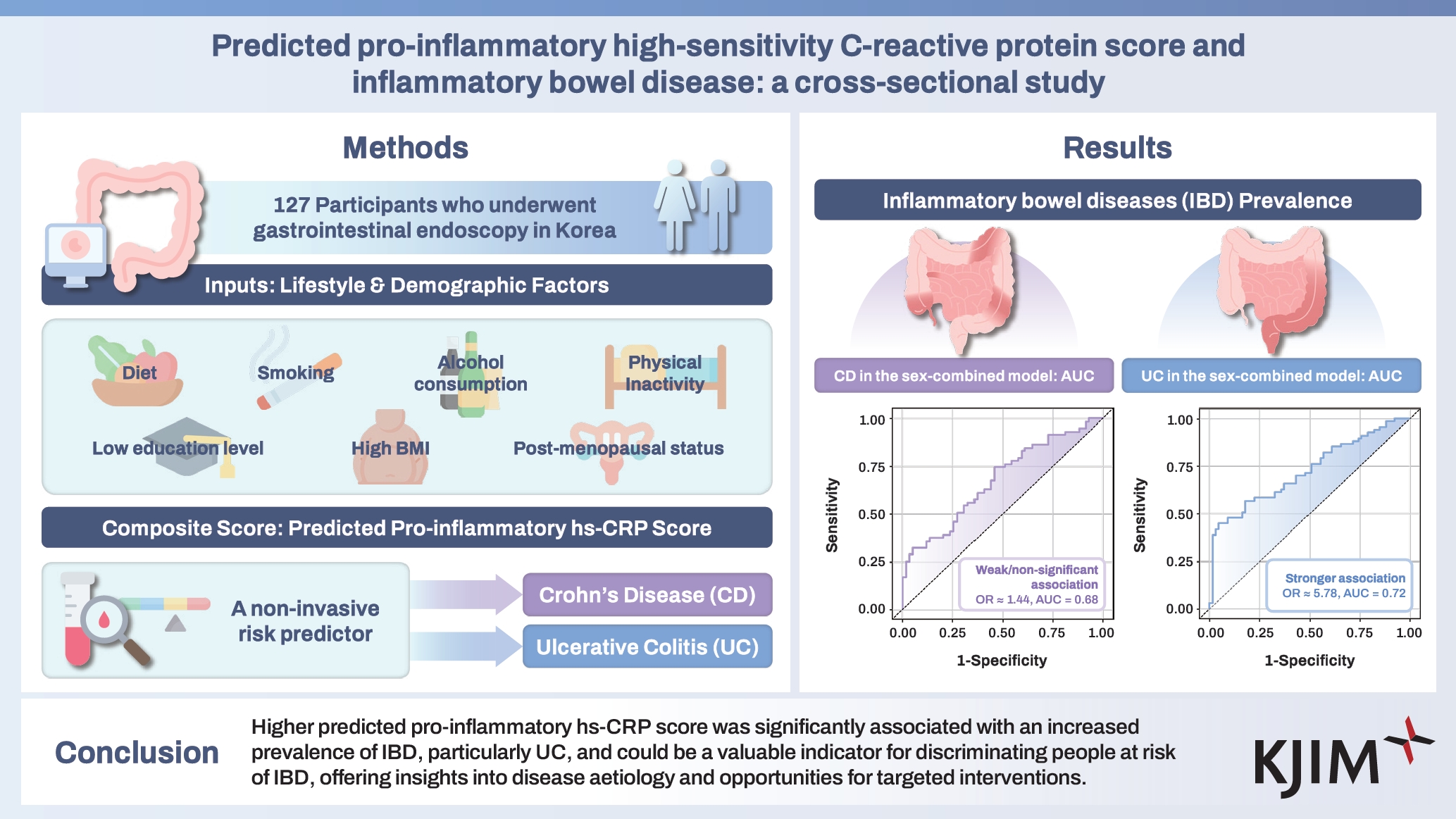
The role of pro-inflammatory factors in the pathogenesis of inflammatory bowel diseases (IBD), is not well understood. This study investigated the association between the predicted pro-inflammatory high-sensitivity C-reactive protein (hs-CRP) score and IBD.
Methods
This study involved 127 case/non-case pairs matched by age and sex of participants who underwent gastrointestinal endoscopy in Korea. Participants provided comprehensive sociodemographic, lifestyle, and dietary data. We obtained odds ratio (OR) and 95% confidence interval (CI) for IBD prevalence by tertiles of the predicted pro-inflammatory hs-CRP score using multivariable-adjusted logistic regression models at a two-sided p < 0.05.
Results
Higher predicted pro-inflammatory hs-CRP score was associated with a higher IBD prevalence; OR (95% CI): 1.00, 0.88 (0.38, 2.07) and 8.11 (2.07, 31.81; p for trend = 0.006). Similar increased trends of IBD prevalence with score increase were observed for men and women. The association was more pronounced for UC prevalence when we separated UC and CD. Compared to the low category, OR (95% CI) were 5.78 (1.29, 25.89) for UC but 1.44 (0.31, 6.69) for CD in the dichotomized higher category. The area under the curve for predicted pro-inflammatory hs-CRP score was 0.72 (95% CI: 0.64, 0.81) for UC and 0.68 (95% CI: 0.58, 0.77) for CD, indicating moderate predictive ability.
Conclusions
Higher predicted pro-inflammatory hs-CRP score was significantly associated with an increased prevalence of IBD, particularly UC, and could be a valuable indicator for discriminating people at risk of IBD, offering insights into disease aetiology and opportunities for targeted interventions.
INTRODUCTION
Inflammatory bowel disease (IBD) encompasses a group of disorders, including ulcerative colitis (UC) and Crohn‘s disease (CD), characterized by chronic inflammation, relapsing and remitting in the gastrointestinal tract [1,2]. UC primarily manifests in the colon, involving the invasion of the mucosa and superficial submucosa, but CD is characterized by its occurrence in both the small and large intestines, with a distinctive feature of transmural involvement [3]. IBD is not limited to gastro-intestines but frequently coexists with extra-intestinal manifestations such as skin and joint disorders and ocular conditions [4].
The prevalence of IBD is on the rise worldwide [5,6], with a surge in pediatric IBD, suggesting that the prevalence of IBD is likely to continue increasing in the foreseeable future [7]. The onset of IBD is influenced by various factors, including genetic predisposition [8,9] and changes in the microbiome composition due to environmental factors [8,10]. Environmental factors, including smoking and the use of medications (such as antibiotics, Non-Steroidal Anti-Inflammatory Drugs, and proton pump inhibitors), encompass a range of pro-inflammatory influences on the microbiome composition and, consequently, influence the development of IBD [11,12]. Furthermore, recent research has reported a close correlation between inflammation, diet and the pathogenesis of IBD [13,14]. Depending on various pro-inflammatory and dietary factors, dysbiosis in gut bacteria may occur, leading to the breakdown of intestinal tight junctions and bacterial translocation, which can exacerbate inflammation [13].
These issues justify the need to design non-invasive approaches for the early identification of populations at risk of IBD for timely intervention. Risk score models derived from sociodemographic and lifestyle characteristics, especially those designed to predict high-sensitivity C-reactive protein (hs-CRP), might be viable and cost-effective options for IBD risk prediction, especially since upregulation of hs-CRP response has been linked to IBD events [15,16]. It is crucial to address gaps in IBD screening and determining the precise non-invasive screening tool for early IBD detection would be viable for early management. Similarly, a non-invasive prediction score based on typical pro-inflammatory lifestyle and dietary factors at the population level could be a valuable screening tool for identifying people at risk of IBD for timely intervention and prevention. Furthermore, deploying a predicted pro-inflammatory hs-CRP score for IBD prevention at the population level could improve public health awareness and promote lifestyle modification and personalized medicine approaches for disease prevention. Overall, these efforts would be valuable for IBD primary prevention.
Several factors, including socioeconomic status [17], physical activity [18], food and dietary consumption [19], and obesity [20], have been linked to the long-term prognosis of chronic inflammation. Earlier, a tested and robust predicted pro-inflammatory hs-CRP score was developed by statistically modelling a constellation of dietary, sociodemographic, lifestyle and reproductive factors associated with inflammation in a nationally representative sample of Koreans [21] and has been associated with the odds of colorectal adenoma [21] and non-alcohol-fatty-liver-disease [22]. However, little is known about the association of the predicted pro-inflammatory hs-CRP score with IBD among Koreans. Assessing the relationship between the predicted pro-inflammatory hs-CRP score and IBD onset among Koreans is likely to extend the scope of understanding the influence of lifestyle, demographic, and anthropometric factors on inflammation in the pathophysiology of IBD. This could help develop a strategy for promoting lifestyle modifications and personalized medicine approaches for the primary prevention of IBD among Koreans. Therefore, this study assessed the relationship between predicted pro-inflammatory hs-CRP score (derived from pro-inflammatory demographic, lifestyle, dietary, and anthropometric factors) and IBD in a Korean population.
METHODS
Study approval and ethical considerations
Data for this current study was from a prospective multi-centre study across eight tertiary medical centres in Korea. Institutional review boards across study sites including, Chonnam National University Hospital (CNUH-2021-250), Chungnam National University Sejong Hospital (CNUSH 2021-08-002), Dongguk University Hospital (DUIH 2021-03-030-005), Kyungpook National University Hospital (KNUH 2021-05-011), Chungbuk National University Hospital (CBNUH 2021-07-027-001), Kangwon National University Hospital (KNUH-A-2021-05-011-012), Eulji University Hospital (EMCS 2022-12-015), and Jeju National University Hospital (2021-06-005). This study followed the ethical principles of medical research involving human participants as outlined in the Declaration of Helsinki. All participants were prospectively enrolled after IRB approval at each study site and provided informed consent before participation in the study.
Study population, data collection and participant selection
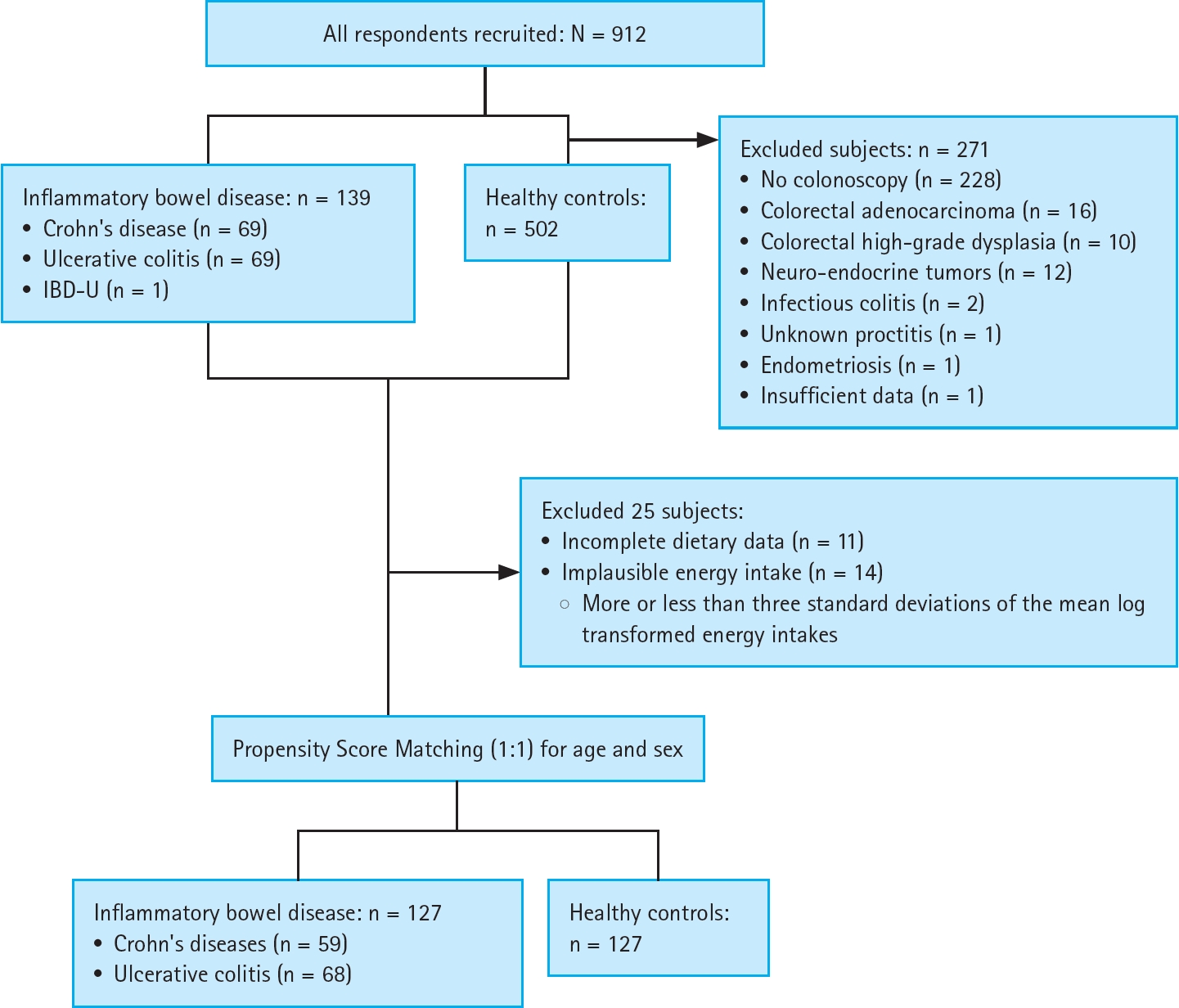
Trained personnel recruited participants from individuals undergoing gastrointestinal endoscopy (esophagogastroduodenoscopy and/or colonoscopy) at the above-mentioned medical centres between July 2021 and September 2023. Comprehensive data encompassing demographic and lifestyle factors, medical history, and metabolic parameters were obtained from medical records. Information on dietary intake was collected using a standardized food frequency questionnaire. From the initial pool of 912 participants, 271 were excluded based on the following criteria: absence of complete colonoscopy information (n = 228), presence of gastrointestinal dysfunction (n = 43), incomplete dietary data (n = 11), and implausible energy intake (defined as an energy intake of more or less than three standard deviations from the mean log-transformed energy intake; n = 14). The remaining IBD patients were matched with IBD-free non-cases on a 1:1 basis according to age and sex using propensity score matching, resulting in a final cohort of 127 case-matched pairs (Fig. 1).
Identification of IBD case
IBD, which includes CD, UC, and unclassified IBD, was diagnosed by trained specialists using a comprehensive diagnostic imaging assessment (including endoscopy, computed tomography, and magnetic resonance imaging), histological examinations, clinical symptom evaluation, blood and faecal test results, medication history and treatment response at each hospital site following standard guidelines [23]. Furthermore, CD and UC were classified according to the Montreal classification for IBD [24].
Evaluation of lifestyle diet and clinical factors
Participants completed questionnaires on their sociodemographics, lifestyle information, and medical histories. Participants reported age (in years) and sex as either ‘male’ or ‘female’. Women’s menopausal status was presented as ‘premenopausal’ or ‘post-menopausal,’ and the highest education completed was reported and classified as ‘elementary to high school’ or ‘college graduate and above’. In addition, current smoking was defined as having smoked a cigarette at least once in the last 12 months. Physical activity in metabolic equivalent tasks (MET-hours/week) was estimated based on the average hours and the number of days spent on moderate to intense levels of physical activity or walking [25] and classified as ‘none’, ‘< 14 MET-hours/week,’ and ‘≥ 14 MET-hours/week’. Body mass index (BMI) was estimated as a function of weight (kg) divided by the square of the height (m), and obesity status was defined as BMI ≥ 25 kg/m2 [26]. Participants provided their dietary intake information using validated food frequency questionnaires. Information on the typical food consumption and portion sizes in the last 12 months preceding the study was reported and documented [27]. There were nine options (ranging from ‘never’ or ‘less than once/month’ to ‘three times/ day’ and three portion size options; ‘one-half of a standard serving’, ‘one standard serving,’ and ‘one and half of standard serving’) for each food and drink item, and average daily consumption of food (in grams/day) and nutrients was determined by multiplying the frequency of consumption with the reported amount. Details of the food frequency questionnaire assessment and food intake estimation have been reported previously [21,28]. Alcohol intake (in grams/day) was estimated as the total ethanol weight, including the multiplication of quantities and frequencies of the types of liquors/alcohol. Also, participants were asked if they had been previously diagnosed with hypertension [29] and diabetes mellitus [30]. Serum levels of hs-CRP were measured in IBD patients using clinical chemical analyzers, specifically the cobas 8000 (Roche Diagnostics System, Basel, Switzerland) and the Beckman Au5821 automatic biochemical analyzer (Beckman Coulter Inc., Brea, CA, USA). The coefficients of variation of these biomarkers were < 2%.
Computation of the predicted pro-inflammatory hs-CRP score
The predicted pro-inflammatory hs-CRP score model used in this study was developed [31] and validated [21] in the Korean population. Briefly, pro-inflammatory factors previously reported to be associated with the blood profiles of hs-CRP in the literature were applied to model the predicted pro-inflammatory hs-CRP score through an empirical constellation of these factors in a linear regression model. These factors include food groups, nutrients, alcohol intake, BMI, smoking status, physical activity, educational level, and menopausal status. The predicted pro-inflammatory hs-CRP score was computed as the total score of the multiplication of the beta coefficients (from sex-combined and sex-specific predicted pro-inflammatory hs-CRP score derived from empirical models) with subject-level pro-inflammatory demographic and lifestyle factors and food intake (grams/day) (Supplementary Table 1). The predicted pro-inflammatory hs-CRP score was categorized into tertiles to include a reasonable number of participants in each category for statistical comparisons.
Statistical analysis
Patients with IBD were matched with IBD-free non-cases by propensity scores. Propensity scores were estimated using a logistic regression model and inverse probability treatment weighting, with age (continuous) and sex (male vs. female) as covariates. IBD cases were matched with IBD-free non-cases in a 1:1 ratio, with the nearest neighbours having propensity scores within the exact boundaries, and unmatched participants were excluded. After matching, the propensity scores and covariates distributions were reviewed to match quality and consistency.
Characteristics of participants by IBD status and across tertile distribution of the predicted pro-inflammatory hs-CRP score were presented using mean ± standard deviation (SD) or median and (range) as applicable for continuous data and frequencies (percentages) for categorical data. Conditional logistic regression models were used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) for the prevalence of IBD according to tertiles of sex-combined and sex-specific predicted pro-inflammatory hs-CRP score. Differences in ORs were assessed when determining the variables to be included in the final model. The regression model in the sex-combined predicted pro-inflammatory hs-CRP scores was adjusted for age (continuous, years), sex (male or female), alcohol intake (continuous, grams/day), smoking (no, past, or current), highest education completed (high school or less, or college education and above), physical activity (continuous, MET-minutes/week), dietary energy intake (continuous, kcal/day), diabetes mellitus status (no or yes), and hypertension status (no or yes). The final regression model of the male-specific predicted pro-inflammatory hs-CRP score was adjusted for the same covariates, excluding sex. Also, the final regression model for the female-specific predicted pro-inflammatory hs-CRP score was adjusted for the same covariates in the sex-combined model and post-menopausal status (no or yes), excluding sex. The median value of the tertile distribution of the predicted pro-inflammatory hs-CRP score was assigned in a continuous model to assess linear trends in the relationship between the predicted pro-inflammatory hs-CRP score and IBD.
In the subgroup analyses by IBD subtype (CD or UC), obesity status (< 25 or ≥ 25 kg/m2), hypertension status (no or yes), and post-menopausal status (no or yes for females), the predicted pro-inflammatory hs-CRP score was dichotomized by median values due to the small sample size. Effect modification was evaluated by including interaction term(s) and comparing the nested models with or without the cross-product using the likelihood ratio test. In addition, the receiver operating characteristic (ROC) curve was plotted in sex-combined and sex-specific regression models by treating the predicted pro-inflammatory hs-CRP score in a continuous model. The area under the curve (AUC) and 95% CI were estimated to determine the predictive ability of the predicted pro-inflammatory hs-CRP score in discriminating IBD. All statistical analyses were conducted using SAS (version 9.4; SAS Institute Inc., Cary, NC, USA), with a two-sided p < 0.05.
RESULTS
Characteristics of participants by IBD status and tertiles of the predicted pro-inflammatory hs-CRP score
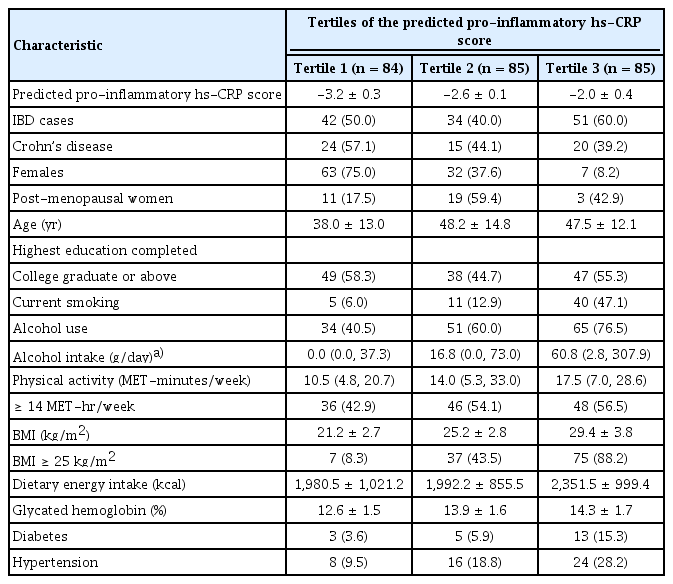
Of the 127 case-non-case pairs of patients with IBD included in this study (Fig. 1), 59 (46.5%) had CD, and 68 (53.7%) had UC. The characteristics, medication history, and results of blood and faecal tests for IBD cases only (including UC and CD) are presented in Supplementary Table 2. Overall, 51 (40.2%) were females, and the median (range) for CRP was 0.1 (0–5.0) mg/dL. Also, the characteristics of the 127 case-non-case pairs are in Supplementary Table 3. Overall, the mean age was 44.6 ± 14.1 years, 102 (40.2%) were females, 56 (22.1%) were current smokers, and 150 (59.1%) reported alcohol use (Table 1). Also, the median (interquartile range) for physical activity was 14.0 (5.3, 28.8) MET-hour/week, the mean BMI was 25.3 ± 4.6 kg/m2, and 119 (46.9%) had a BMI of at least 25 kg/m2. Mean energy intake was 2,108.6 ± 972.9 kcal/day, mean glycated haemoglobin was 13.6 ± 1.7%, 21 (8.3%) had diabetes mellitus, and 48 (18.9%) were hypertensives.
When the characteristics of participants were stratified by IBD status (Supplementary Table 3), age and sex did not differ, but IBD patients vs. non-IBD cases presented a lower prevalence of current smoking (19.7% vs. 24.4%, respectively), alcohol use (48.0% vs. 70.1%, respectively), and hypertension (17.3% vs. 20.5%, respectively). In contrast, the prevalence of obesity (58.3% vs. 35.4%) was higher among IBD patients than non-IBD cases. In Table 1, participants in the third tertile of the predicted pro-inflammatory hs-CRP score presented with a higher prevalence of IBD, current smoking, alcohol use, obesity, diabetes mellitus, and hypertension, but a lower proportion of females compared to those in the first tertile of the predicted pro-inflammatory hs-CRP score. This trend was similar to the sex-specific models of the pro-inflammatory predicted hs-CRP score (Supplementary Table 4).
Predicted pro-inflammatory hs-CRP score and odds of IBD
The ORs and 95% CIs of IBD prevalence by tertile distribution of the predicted pro-inflammatory hs-CRP score are presented in Table 2. In the age-adjusted conditional logistic regression model, a higher predicted pro-inflammatory hs-CRP score was associated with a higher prevalence of IBD: 1.00, 0.81 (0.38, 1.72), and 2.66 (1.08, 6.54; p for trend = 0.02). The ORs were robust when the model was additionally adjusted for sex, alcohol intake, current smoking status, education, physical activity, dietary energy intakes, diabetes mellitus status and hypertension status; 1.00, 0.88 (0.38, 2.07) and 8.11 (2.07, 31.81; p for trend = 0.006). A similar but statistically insignificant trend was observed for the male-specific predicted pro-inflammatory hs-CRP score: 1.00, 2.09 (0.61, 7.16), and 3.65 (0.85, 15.67; p for trend = 0.08) and female-specific predicted pro-inflammatory hs-CRP score; 1.00, 4.07 (0.82, 20.13), and 2.88 (0.48, 17.42; p for trend = 0.25).

Odds ratios and 95% confidence intervals for IBD according to the tertiles of the predicted pro-inflammatory hs-CRP score for the sex-combined, male-specific, and female-specific models
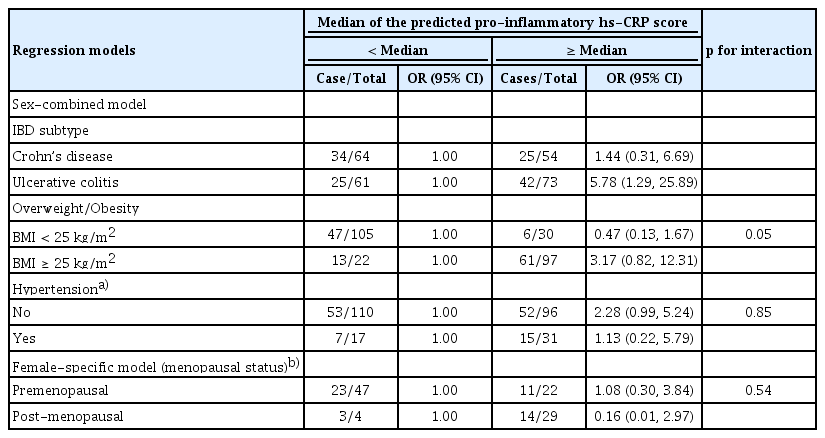
We dichotomized the sex-combined predicted pro-inflammatory hs-CRP scores for the subgroup analysis by IBD subtype, obesity, hypertension, and menopausal status (for the female-specific model only) – Table 3. A higher predicted pro-inflammatory hs-CRP score was associated with a higher prevalence of UC [< median – 1.00 and ≥ median – 5.78 (1.29, 25.89)], but not for CD [< median – 1.00 and ≥ median – 1.44 (0.31, 6.69)]. A similar trend was observed in the overall sample; however, a higher predicted pro-inflammatory hs-CRP score was not significantly associated with a higher prevalence of IBD, independent of obesity, hypertension, or menopausal status, with no evidence of interaction (Table 3).
The area under the ROC curve of the predicted pro-inflammatory hs-CRP score
Furthermore, the predicted pro-inflammatory hs-CRP score was treated as a continuous variable to evaluate its ability to classify IBD (Fig. 2), and the AUC (95% CI) was 0.67 (0.61, 0.74) for the sex-combined model, 0.71 (0.63, 0.80) for the male-specific model, and 0.65 (0.54, 0.75) for the female-specific model. Similarly, the AUC (95% CI) results for the IBD subtype were 0.68 (0.58, 0.77) for CD and 0.72 (0.64, 0.81) for UC, thereby suggesting that the predicted pro-inflammatory hs-CRP score possibly demonstrated a modest predictive ability in discriminating IBD with a portentous likelihood of viability for population-level risk screening to identify at-risk groups for early timely intervention.

Receiver-operating characteristic (ROC) for IBD according to the predicted pro-inflammatory hs-CRP scores in the sex-combined model (A), Crohn‘s disease in the sex-combined model (B), ulcerative colitis in the sex-combined model (C), male-specific model (D), and female-specific model (E). Models included age (continuous, years), sex (males or females), alcohol intake (continuous, g/day), smoking (no, past or current), highest education completed (high school and less, or college education and above); physical activity (continuous, MET-minutes/week), energy intakes (continuous, kcal/day), diabetes mellitus status (no or yes) and hypertension status (no, yes). However, sex (males/females) as a covariate was excluded in the sex-specific (male and female) regression models. IBD, inflammatory bowel disease; hs-CRP, high-sensitivity C-reactive protein; MET, metabolic equivalent task; AUC, area under the curve; CI, confidence interval. a)Models included age (continuous, years), alcohol intake (continuous, g/day), smoking (no, past or current), highest education completed (high school and less, or college education and above); physical activity (continuous, MET-minutes/week), energy intakes (continuous, kcal/day), post-menopausal status (no or yes), diabetes mellitus status (no or yes) and hypertension status (no, yes).
DISCUSSION
In this study, we found that a higher predicted pro-inflammatory hs-CRP score was associated with a higher prevalence of IBD, especially UC, but not CD. The predicted pro-inflammatory hs-CRP scores demonstrated distinct patterns of associations by IBD subtype, suggesting viable information for public health interventions and programming for the primary prevention of IBD. Our findings demonstrate the viability of using known demographic and lifestyle factors in a prediction model to discern the onset of inflammation as a nexus for identifying populations at risk of disease for timely intervention and prevention.
Pro-inflammatory indexes, especially from diet intake only, have been linked to health outcomes across diverse populations [32-34]. The predicted pro-inflammatory hs-CRP score in this current study was unique given that it was derived using information not limited to diet only but also multiple typical factors such as demographic, lifestyle, and anthropometric factors, suggesting its viability as a reliable tool for identifying populations at risk of poor health outcomes, including IBD. Previously, the predicted pro-inflammatory hs-CRP scores were promising for discriminating colorectal adenoma [21] and non-alcoholic fatty liver disease [22]. In addition, several reports have demonstrated the associations of the pro-inflammatory index with cardiovascular events and several cancers [35-37], with the scarcity of evidence on the viability of the predicted pro-inflammatory hs-CRP scores in IBD events. Our study contributes significantly to the growing body of literature on the significance of dietary and lifestyle factors in IBD epidemiology. It offers unique approaches for the early identification of at-risk populations for early prevention and management.
Furthermore, feasible explanations exist for the positive association between higher predicted pro-inflammatory hs-CRP score and IBD risk. First, subclinical long-term inflammation has been linked to IBD manifestations [15,16] and prognosis [38], independent of subtypes [39]. Given that the current predicted pro-inflammatory hs-CRP score was designed from a constellation of pro-inflammatory lifestyle and dietary factors to predict inflammation, the observed association can be linked to the capacity of these pro-inflammatory factors to trigger subclinical but chronic inflammation, which might present asymptomatically [40] that can lead to IBD onset. We found a significant association for UC even with a low sample size, meaning a stronger association for UC than CD. The fact that higher predicted pro-inflammatory hs-CRP scores was associated with the odds of UC, but not CD in this study can be explained in several ways. Pathophysiological differences in the inflammatory responses in the bowel may explain these associations. There is modest evidence suggesting that patients with IBD more often present with normal-range blood hs-CRP levels, mainly by subtype [16]. This inevitably limits the significance of blood hs-CRP levels in accurately discriminating IBD subtypes. However, this study’s predicted pro-inflammatory hs-CRP scores overcomes such limitations, which lends credence to its use as a soft target tool for identifying at-risk population groups for timely interventions.
However, the closer association of the predicted pro-inflammatory hs-CRP scores with UC than CD in this study did not align with a few other studies suggesting a positive association with CD. Empiric Dietary Inflammatory Pattern score [41] or ultra-processed food intake [42,43] was more strongly associated with CD than UC in cohort studies. The reasons are not entirely clear, but we hypothesize that the complex interplay of dietary exposure and genetic differences could explain it. For instance, it has been established that long-term dietary patterns are associated with the pro-inflammatory and anti-inflammatory features of the gut [44]. Most of these existing studies were from Western populations, with limited data from Asian populations. Similarly, genetic differences exist between Western and Eastern populations concerning IBD [45] and significant differences in dietary patterns [42]. Additionally, there are differences in the incidence and prevalence patterns of UC and CD between these populations [6]. Given these distinctions, it is plausible that genetic and dietary factors specific to the population studied in our research could explain the higher association of predicted pro-inflammatory hs-CRP scores with UC compared to CD. The unique genetic background and nutritional habits of the population might influence the inflammatory response differently, leading to the observed results. Further research on diverse populations is necessary to understand these differences and their implications for IBD. Also, given its association with IBD prevalence, the predicted pro-inflammatory hs-CRP scores could support clinicians’ efforts in making a case for lifestyle modification to counter pro-inflammatory sociodemographic, lifestyle and dietary factors, thereby improving IBD patients’ care, quality of life and management, in addition to reducing the burden of IBD. Similarly, our findings offer evidence-based information to guide health personnel in promoting public health awareness for healthy lifestyles and viable dietary choices for the primary prevention of IBD.
This study has some inherent limitations. First, this is a cross-sectional study, and causal inference cannot be implied. Measurement errors typical of most epidemiological studies cannot be ruled out. The sample size was relatively small, implying low statistical power to detect a difference. A few IBD patients in the course of recruitment might have provided information on dietary intake likely after the onset of symptoms such as diarrhoea, bloody stools, and abdominal pain pre- or post-IBD diagnosis, which might influence the report of their usual diet. The correlation between the predicted pro-inflammatory hs-CRP scores and serum CRP concentration was untested due to the shortage of data among non-IBD cases. Also, our findings must be interpreted with caution in light of other important factors, including but not limited to in-utero exposures, environmental exposures, stability of lifestyle habits, genetic predilection, and gut and microbiome diversities, among others that were unaccounted for in modelling the pro-inflammatory hs-CRP scores to discriminate IBD events. However, our study has several strengths. Gastroenterologists performed colonoscopies to ascertain IBD, CD, and UC, lending credence to the objectivity of case determination. The objective constellation of pro-inflammatory dietary and lifestyle factors for the predicted pro-inflammatory hs-CRP scores is a strength of this study. In addition, the regression models were adjusted for potential confounding factors, including age, sex, alcohol intake, smoking, highest education completed, physical activity, energy intake, diabetes mellitus, and hypertension, to discern whether these factors could manipulate the association of the predicted pro-inflammatory hs-CRP scores with IBD. Similarly, subgroup analyses by IBD subtype, obesity, hypertension and menopausal status were carried out to discern whether these factors could manipulate the association of the predicted pro-inflammatory hs-CRP scores with IBD. Because there were fewer diabetes cases, a subgroup analysis by diabetes status was not carried out.
In conclusion, we found that higher predicted pro-inflammatory hs-CRP scores were associated with higher odds of IBD, especially UC. Our study suggests that predicted pro-inflammatory hs-CRP scores could be a valuable indicator for identifying individuals at higher risk for IBD, highlighting the potential importance of lifestyle and demographic factors in the pathogenesis of these conditions.
KEY MESSAGE
1. Higher predicted pro-inflammatory hs-CRP scores were associated with a higher prevalence of IBD, especially UC, but not CD.
2. The predicted pro-inflammatory hs-CRP scores also demonstrated distinct patterns in discriminating IBD, indicating its prognostic potential for the population- level risk evaluation of IBD.
3. Our findings demonstrate the viability of using known demographic and lifestyle factors in a prediction model to discern the onset of inflammation as a nexus for identifying populations at risk of IBD for timely intervention and prevention.
Notes
Acknowledgments
We thank the staff and volunteers from multiple hospitals for recruiting participants for this study.
CRedit authorship contri
Dong Hyun Kim: conceptualization, methodology, data curation, writing - original draft, visualization; Akinkunmi Paul Okekunle: conceptualization, methodology, investigation, data curation, formal analysis, validation, writing - original draft, visualization; Jioh Kang: methodology, data curation, formal analysis, validation, software, writing - review & editing; Hyun-Soo Kim: methodology, writing - review & editing, project administration; Sang Hoon Kim: methodology, investigation, data curation, writing - review & editing, project administration; Min- Kyu Jung: methodology, investigation, data curation, writing - review & editing, project administration; Jae Ho Park: methodology, investigation, data curation, writing - review & editing, project administration; Soo Young Na: methodology, investigation, data curation, writing - review & editing, project administration; Hoonjai Chun: methodology, investigation, data curation, writing - review & editing, project administration; Jung Eun Lee: conceptualization, methodology, resources, validation, writing - review & editing, visualization, supervision, project administration, funding acquisition; Yun Jeong Lim: conceptualization, methodology, investigation, data curation, writing - review & editing, visualization, supervision, project administration, funding acquisition
Conflicts of interest
The authors disclose no conflicts.
Funding
This work was supported by national grants from the Ministry of Science and ICT, National Research Foundation of Korea (2022R1F1A1066166 and 2020H1D3A1A04081265), Korean Journal of Gastrointestinal Cancer Research Foundation 2022, and Chonnam National University Hospital Biomedical Research Institute (BCRI25090 and BCRI25028).