Diagnostic approach for incidental pulmonary nodules
Article information
Abstract
Lung cancer remains a leading cause of cancer-related mortality worldwide and is often diagnosed at an advanced stage, with poor survival outcomes. Early detection and appropriate management of incidental pulmonary nodules, frequently identified through low-dose computed tomography screening, are critical for improving prognosis and reducing lung cancer mortality. Established guidelines, including those of the Fleischner Society and American College of Radiology, provide structured recommendations for risk assessment, surveillance, and intervention. Recent advancements in diagnostic modalities, such as positron emission tomography, endobronchial ultrasound, electromagnetic navigation bronchoscopy, and robot-assisted bronchoscopy, have enhanced the diagnostic accuracy while minimizing procedural risks. A multidisciplinary approach that incorporates these technologies is essential for optimizing patient care. This review summarizes the current strategies for evaluating and managing solitary pulmonary nodules, including risk stratification models, imaging features, and biopsy techniques, thereby providing a comprehensive overview for clinicians.
INTRODUCTION
Lung cancer is a leading cause of cancer-related mortality worldwide [1]. It is often diagnosed at an advanced stage and recurs even after curative-intent surgery. The prognosis of non-small cell lung cancer (NSCLC) is directly related to its stage at diagnosis, with a 5-year survival rate ranging from 92% in stage IA1 to 0% in stage IVB [2]. Despite significant advancements in treatment modalities, including targeted therapy and immunotherapy, the overall 5-year survival rate of lung cancer patients remains at approximately 18% [3]. This underscores the critical importance of early detection and intervention, which offer the best chance for a favorable outcome. The effectiveness of the early detection of lung cancer has been demonstrated in large-scale clinical trials. In the NELSON trial, 59.6% of screen-detected lung cancers were diagnosed as stage IA or IB, whereas only 9.4% were detected as stage IV, highlighting a significant stage shift toward earlier, more treatable disease [4].
Early screening efforts using chest radiography, sputum cytology, and various tumor markers have failed to improve lung cancer mortality [5–7]. However, the introduction of low-dose computed tomography (LDCT) in high-risk individuals aged ≥ 55 has revolutionized lung cancer screening, demonstrating a 20% reduction in lung cancer mortality compared with chest radiography [8]. Since then, LDCT-based lung cancer screening has been implemented on a large scale in many countries, leading to a substantial increase in the detection of pulmonary nodules.
The increase in incidental pulmonary nodule detection poses new challenges for optimal management, including appropriate follow-up, diagnostic evaluation, and treatment strategies. This review aims to provide a comprehensive and updated overview of the diagnostic approach for solitary pulmonary nodules (SPNs), with a focus on evidence-based management strategies to enhance clinical decision-making.
DEFINITION
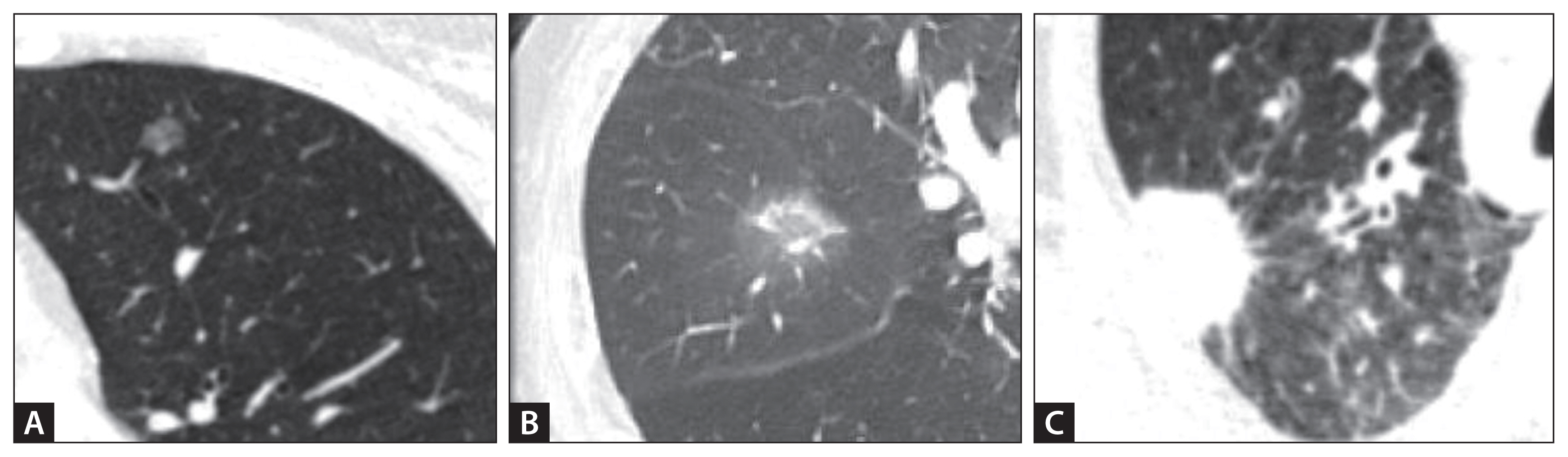
An SPN is defined as a single well-circumscribed lesion in the lung, typically measuring < 30 mm in diameter, surrounded by normal lung parenchyma without associated atelectasis, lymphadenopathy, or pleural effusion [9]. Lesions > 30 mm are classified as lung masses rather than nodules and are more likely to be malignant [9]. Morphologically, pulmonary nodules are categorized as solid or subsolid, with subsolid nodules further subdivided into pure ground-glass and partially solid nodules (Fig. 1). Although solid nodules are more frequently encountered, part-solid nodules have a higher probability of malignancy and often exhibit indolent growth patterns [10]. Understanding morphological characteristics is essential for risk stratification and guiding appropriate diagnostic and management strategies.
EPIDEMIOLOGY
The detection rate of pulmonary nodules during lung cancer screening varies between studies. According to the National Lung Screening Trial, which targeted high-risk individuals aged 55–74 years with a smoking history of at least 30 pack-years, approximately 27% of pulmonary nodules were detected by LDCT, whereas the detection rate was only approximately 7% when chest radiography alone was used [8]. In a follow-up study, the NELSON trial reported that the detection rate of pulmonary nodules ranged from approximately 25% to 50%, with variability based on age, smoking history, and regional characteristics of the screened subjects [4]. The majority of detected pulmonary nodules were small, measuring 4–10 mm, and most were benign, whereas malignant pulmonary nodules accounted for approximately 5–10% of cases. In addition, the incidence of newly detected pulmonary nodules in repeated lung cancer screenings was reported to be approximately 3–6% [11].
SPNs can be classified as either benign or malignant, with the estimated prevalence varying across studies depending on the study population and diagnostic confirmation method [12]. Screening studies of smokers at high risk of malignancy have shown that most nodules detected by CT are benign. For example, only 144 (1%) of 12,029 nodules identified in the Pan-Canadian Early Detection of Lung Cancer and British Columbia Cancer Agency studies were malignant [10]. Common causes of benign lung nodules include transient infectious diseases, benign tumors such as granulomas and hamartomas, benign vascular diseases such as arteriovenous malformations, intrapulmonary lymph nodes, and benign nodules such as sarcoidosis. Differentiating between benign and malignant nodules is essential for appropriate clinical management and the prevention of unnecessary invasive procedures.
INITIAL EVALUATION
The diagnostic evaluation of SPNs involves a comprehensive approach that integrates clinical assessment and radiological findings. Depending on risk stratification, management may include CT surveillance or further diagnostic procedures, such as tissue sampling.
Clinical assessment
The initial step in evaluating an SPN is to obtain a detailed medical history, with a focus on the risk factors for malignancy. Smoking is the most important risk factor, with malignancy risk escalating in proportion to the smoking duration and intensity [13]. Other key risk factors include older age, particularly over 50 years of age [14], and a family history of lung cancer, especially in first-degree relatives [15]. Occupational and environmental exposure to carcinogens such as asbestos, radon, and other hazardous chemicals significantly increases the likelihood of malignancy [16]. In terms of clinical manifestations, most SPNs are asymptomatic and are incidentally detected during imaging studies; however, the presence of symptoms such as hemoptysis, weight loss, and fatigue may suggest a higher probability of malignant tumors and necessitate further evaluation.
Risk assessment for malignancy
Clinicians can use validated clinical prediction models that integrate multiple risk factors to estimate the likelihood of malignancy. The Brock model incorporates variables such as sex, age, nodule size, family history of cancer, emphysema, number of nodules, solid components, upper lobe involvement, and bed parameters [10]. The risk probability is classified as low if it is < 5%, moderate if it is between 5% and 65%, and high if it is > 65%. The Mayo model considers age, nodule size, smoking history, history of cancer without 5-year restrictions, upper lobe involvement, nodule spiculation, and positron emission tomography (PET) scan information [17]. Although there are several old models, no single superior predictive model exists. Therefore, clinical judgment, in conjunction with imaging features, is crucial for estimating the malignancy risk.
CT imaging characteristics
Nodules on CT can be distinguished by their size, degree of attenuation, growth, calcification pattern, spiculated borders, and enhancement.
Nodule size is an independent predictor of malignancy [10,18]. The risk of malignancy increases with size: nodules < 5 mm: < 1%, nodules 5–9 mm: 2–6%, nodules 8–20 mm: 18%, and nodules ≥ 20 mm: > 50%. Nodule attenuation allows for the classification of lesions as solid or subsolid (pure ground-glass or part-solid). Partially solid lesions are more likely to be malignant [10].
Growth in solid nodules is defined as an increase in size of ≥ 2 mm, whereas in subsolid nodules, it includes an increase in attenuation, size, or development of a solid component [19]. In addition, studies evaluating the volume doubling time (VDT) of tumors are helpful in predicting the malignant potential of lung nodules. Malignant nodules typically have a VDT of 20 to 400 days, with longer VDT (> 400 days) observed in in situ adenocarcinoma and minimally invasive adenocarcinoma [20].
Spiculation is attributed to the growth of malignant cells along the pulmonary interstitium and lobulation to differential growth rates within the nodules. Typically, benign nodules have well-defined smooth borders, whereas malignant nodules have spiculated or lobular borders [18]. Calcification patterns can be used to reliably diagnose incidental pulmonary nodules as benign, central, diffuse, lamellated, or popcorn nodules. Indeterminate patterns of calcification (e.g., punctate, eccentric, and amorphous) are nonspecific, and a nodule containing one of these patterns may be malignant. According to enhancement, nodules enhancing < 15 Hounsfield unis are likely benign, whereas malignant nodules typically enhance > 20 Hounsfield units [21].
NODULE FOLLOW-UP INCLUDING CT SURVEILLANCE
Several clinical guidelines, including those from the Fleischner Society, American College of Chest Physicians (ACCP), and American College of Radiology (Lung Reporting and data system [Lung-RADS]), provide recommendations for evaluating incidentally detected and screen-detected pulmonary nodules.
Fleischner Society recommendations
The Fleischner Society offers detailed follow-up strategies based on the nodule size and individual risk factors. For instance, small nodules (< 6 mm) may require minimal follow-up in low-risk patients, whereas larger or irregular nodules may require close monitoring or further diagnostic evaluation (Table 1) [9].
ACCP
The ACCP provides a risk-based management approach that stratifies patients into low-, intermediate-, and high-risk groups. It recommends surveillance imaging for low-risk nodules, biopsy or advanced imaging for intermediate-risk nodules, and definitive surgical evaluation for high-risk nodules (Table 2) [22].
American College of Radiology (Lung-RADS)
The Lung-RADS standardizes the reporting of LDCT findings and is widely used [23]. Radiologists assign a Lung-RADS score (LR 0–4) that considers lung nodules and other findings associated with lung cancer (e.g., airway nodules and atypical cystic lesions), potentially inflammatory/infectious lesions, and other important incidental findings. Each LR score correlates with the lung cancer risk. The primary factors considered in the LR score are nodule consistency, size, and growth, along with other nodule factors, including peripheral and juxtapleural nodule locations and bed margins. Based on this risk, recommendations are made for further management. The LR scores are periodically revised; the most recent version is the Lung-RADS 2022 (Table 3). The Lung-RADS defines LR-1 and LR-2 as “negative” and LR-3 and LR-4 as “positive” CT screening results.
Generally, most small nodules (6–8 mm) can be managed with periodic CT surveillance according to guideline recommendations. Lesions > 30 mm without benign features have high malignant potential and can be resected without biopsy because the benefits of resection outweigh the risks associated with surgery. Nodules 8–30 mm in size present varying clinical scenarios regarding whether to continue surveillance or proceed with biopsy, requiring individualized decision-making based on malignancy potential and procedural risks.
Although specific guidelines may differ slightly among academic societies, institutions should consider adopting standardized protocols tailored to their patient populations and incorporating one or a combination of these guidelines for optimal management.
ROLE OF ARTIFICIAL INTELLIGENCE IN SPNS
Recent advancements in artificial intelligence, particularly deep learning (DL), have resulted in significant transformations in the field of medicine, especially in radiology. DL technologies are effectively used to detect and classify lesions, as well as to quantify both normal and abnormal anatomical structures. In the domain of lung nodules and lung cancer, DL algorithms have demonstrated performance comparable to that of radiologists in quantifying the solid components of lung adenocarcinomas and distinguishing their invasiveness [24,25]. Moreover, DL has been effectively applied to classify lung nodules as benign or malignant, estimate their growth rates, and assess the risk of lung cancer development [26,27].
PET-CT
PET-CT plays a crucial role in the evaluation of SPNs, either when incidentally detected or as a follow-up to findings from other imaging modalities. However, PET-CT has inherent resolution limitations, particularly for nodules with a solid component < 8 mm, where tracer uptake may not be reliably assessed. A meta-analysis of PET-CT results demonstrated a sensitivity of 89% (95% confidence interval [CI], 86–91%) and specificity of 75% (95% CI, 71–79%) [28]. False-negative results are more likely in nodules with solid components ≤ 8 mm and in pure ground-glass nodules, as these lesions often exhibit low metabolic activity on fluorodeoxyglucose PET-CT [29]. Additionally, certain histological subtypes of lung cancer, including intraepithelial carcinoma, minimally invasive adenocarcinoma, mucinous adenocarcinoma, and carcinoid tumors, may exhibit reduced fluorodeoxyglucose uptake, contributing to false-negative findings. Conversely, false-positive results are common in infectious and inflammatory conditions, including granulomatous inflammatory diseases and rheumatoid nodules.
TISSUE DIAGNOSIS
Non-surgical biopsy
Non-surgical biopsies can be performed using bronchoscopic or transthoracic needles. The choice of sampling procedure depends on multiple factors, including the size and location of the nodule, availability of the procedure, and institutional expertise. Conventional bronchoscopic techniques are preferred for larger, more centrally located lesions, whereas transthoracic needle biopsy techniques are preferred for smaller, more peripheral lesions. Advanced bronchoscopic approaches (e.g., virtual bronchoscopy, electromagnetic navigation, radial ultrasound, and robotic bronchoscopy) have improved the diagnostic yield for small peripheral nodules.
Transthoracic needle biopsy
Fluoroscopy- and CT-guided lung biopsies are widely used to diagnose SPNs. Owing to the high radiation exposure associated with conventional CT-guided biopsy and the disadvantages of fluoroscopic guidance, cone-beam CT is increasingly being used for lung biopsy. The two main types of percutaneous biopsy are fine-needle aspiration and core biopsy. While both methods have similar diagnostic accuracies for malignant lesions, they differ in the amount of tissue obtained and incidence of complications; therefore, the judgment of an experienced operator is considered important.
Transthoracic needle biopsy demonstrates high sensitivity (> 90%), specificity (> 99%), and diagnostic yield (> 90%) for malignancy, even in nodules < 1 cm [30–34]. However, this procedure is risky. The main complication of image-guided lung biopsy is pneumothorax, occurring in 0–61% of lung biopsies [30,31]. However, additional interventions, such as thoracostomy, are required in approximately 7% of cases. The risk of pneumothorax is related to the length of lung parenchyma passed, patient age, presence of emphysema, needle diameter, and location of the needle through the aerated lung, and it increases significantly with the number of manipulations and/or lesions. Pulmonary hemorrhage is another common complication, reported in 5–16.9% of patients, and may occur concurrently with hemoptysis in 1.25–5% of patients [30,35,36]. Air embolism, although rare, can lead to severe consequences such as stroke, myocardial infarction, or death, if not promptly recognized. Tumor seeding along the needle tract is an extremely rare complication of CT-guided lung biopsy, with a reported incidence of 0.02–0.39% [37–39].
Bronchoscopic techniques
Several bronchoscopic techniques are available to facilitate SPN biopsies. However, their use is generally limited to centers with specialized equipment and requires experienced operators with knowledge of these techniques. The diagnostic yield ranges from 50% to 88% (average, 74%) and generally depends on various factors, including the size and location of the lesion, equipment used, and biopsy technique (Table 4).
Conventional bronchoscopic biopsy or transbronchial needle aspiration has a reported sensitivity for SPNs ranging from 65% to 88%, with higher sensitivity for large central lesions and lower rates for peripheral nodules (> 2 cm, 63%; < 2 cm, 34%) [40,41]. Although less invasive methods for obtaining tissue (washing, lavage, or brushing) can occasionally be used to diagnose malignancy, they are unlikely to obtain enough tissue for immunohistochemical or genetic analysis.
A systematic review of 18 studies found that fluoroscopy-guided endobronchial needle aspiration had a higher diagnostic yield than blind transbronchial lung biopsy (TBLB) (60% vs. 45%) [42]. Compared with fluoroscopy, high-resolution CT imaging during bronchoscopy can provide real-time images to guide the bronchoscope/instruments directly into the target lesion. However, a randomized trial comparing conventional bronchoscopy with CT-guided bronchoscopy for the diagnosis of lung cancer in peripheral lesions and lymph nodes demonstrated no significant difference in the diagnostic yield [42]. Additionally, this technique is not widely used because it is difficult to make appointments in a CT laboratory and requires significant radiation exposure.
Ultrathin bronchoscopy
Ultrathin bronchoscopes have a smaller diameter than conventional bronchoscopes, with a diameter of 2.8–3.2 mm and a working channel of 1.2–1.7 mm. Compared with conventional endoscopes, ultrathin bronchoscopes can reach up to the ninth branch, allowing closer access to the surrounding lung lesions, thereby maximizing proximity to the lesion and improving the diagnostic yield. In a multi-center randomized study of peripheral lung nodules (median diameter, 19 mm), the diagnostic yield was significantly higher with a 3.0-mm ultrathin bronchoscope than with a 4.0-mm bronchoscope (74% vs. 59%, p = 0.04) [43].
Advanced bronchoscopic procedures
Recent advancements in bronchoscopic techniques, including CT reconstruction-based navigation, electromagnetic navigation bronchoscopy (ENB), endobronchial ultrasound (EBUS), and robotic bronchoscopy, have improved diagnostic yields compared with fluoroscopy-guided bronchoscopy alone (Table 4, 5). However, these specialized techniques are only available in select centers and require specific training for their proper use.

List of studies examining the diagnostic yield of various techniques for the diagnosis of lung nodules
Furthermore, Frozen biopsy is gaining attention for lung cancer diagnosis because of its ability to collect larger tissue samples for additional testing beyond conventional pathology. The development of a 1.1 mm cryoprobe has enabled frozen biopsy in radial EBUS (R-EBUS)-guide sheath-TBLB procedures. It allows the sampling of lesions near the bronchial tubes but carries an increased bleeding risk. Retrospective studies have shown that performing frozen biopsy after conventional biopsy increases the diagnostic yield by 14.5% to 29.9%. Matsumoto et al. [44] found that cryobiopsy provided a higher additional diagnostic yield in lesions with negative bronchial signs (15.4%) than in those with positive signs (6.3%).
R-EBUS transbronchial needle aspiration with or without guide sheath
R-EBUS advances rotating ultrasound through the working channel of the bronchoscope to generate 360-degree ultrasound images of peripheral lung nodules beyond the bronchoscope scope, allowing real-time localization. After confirming the exact location, a guide sheath and scope are positioned, the ultrasound probe is removed, and tissue samples are collected using brush cytology or biopsy forceps. The use of a guide sheath improves the diagnostic accuracy, particularly when combined with TBLB forceps.
Although R-EBUS devices tend to be more cost-effective than ENB systems, the efficient use of radial probes and interpretation of peripheral pulmonary ultrasound images require extensive training. To improve success rates, Kurimoto and Morita proposed a technique for reading CT scans and preparing a pre-procedure roadmap. This approach involves careful analysis of the bronchial tree on CT images, allowing clinicians to visualize and map the pathway to the target lesion. In a cohort study involving 1,143 cases, this manual mapping technique significantly improved the diagnostic sensitivity of bronchoscope brushing for malignant nodules compared with conventional brushing methods [45].
A comprehensive systematic review and meta-analysis of 51 studies with 7,601 participants found that R-EBUSTBLB had a pooled sensitivity of 72% (95% CI, 70–75%). The area under the receiver operating characteristic curve was calculated as 0.96 (95% CI, 0.94–0.97), and the risk of pneumothorax was relatively low at 0.7% (95% CI, 0.3–1.1%) [46]. The diagnostic yield was notably higher in cases where the lesion exceeded 2 cm in size, was malignant, and was linked to the airway (bronchial sign), or when R-EBUS imaging showed concentric patterns, confirming that the probe was positioned within the lesion.
Virtual bronchial navigation (VBN)
VBN reconstructs CT images to guide bronchoscopy for peripheral lung lesions. However, a key limitation is the potential discrepancy between the pre-procedure CT-based navigation and the real-time location of the lesion. The currently available VBN systems include the Bf-NAVI/DirectPath (Cybernet Systems, Tokyo, Japan), LungPoint VBN system (Broncus Medical, San Jose, CA, USA), and Synapse 3D system (Fujifilm, Tokyo, Japan).
The diagnostic yield of VBN ranges from 67 to 80%, primarily from expert centers. A pooled analysis of 13 studies found an overall yield of 74% [47], with a lower accuracy (67%) for lesions ≤ 2 cm. VBN is often used in conjunction with EBUS and fluoroscopy to enhance the diagnostic efficacy. A meta-analysis of 39 studies reported a diagnostic yield of 72%; however, many procedures combined VBN with other image-guided biopsy techniques [48]. Randomized clinical trials comparing VBN-assisted and non-VBN-assisted techniques have yielded mixed results. One study showed an improved diagnostic yield with VBN (80% vs. 67%) [49], whereas another found no significant difference (67% vs. 60%) [50].
ENB
ENB can be likened to a real-time “GPS” update on the VBN-generated “map.” It is a medical technology that guides the path in real time by identifying the position of the endoscopic tool or catheter and generating a low-intensity electromagnetic field around the patient’s chest using a magnetic field and generator. However, similar to VBN, ENB is limited by potential discrepancies owing to respiratory motion, atelectasis, and CT-reconstructed pathway deviations. It relies on CT-reconstructed mapping, so there may be differences in the actual path, and magnetic imaging has a technical disadvantage in that it can lead to discrepancies due to respiratory movement and atelectasis. The ACCP recommends ENB for evaluating nodules with an intermediate risk of malignancy. The diagnostic yield of ENB ranges from 44% to 75%, with an average of approximately 65% [22,51–53]. When combined with R-EBUS, the diagnostic yield significantly improves to 88% compared with 59% for ENB alone and 69% for EBUS alone [54]. The large international NAVIGATE study, which included 1,215 patients, reported a 94% tissue acquisition success rate, with a 73% diagnostic yield at 12 months, and malignancy was detected in 44% of patients [55,56]. Complication rates are generally low, with pneumothorax occurring in 5% of cases (2.9% requiring chest tube placement), bronchopulmonary hemorrhage in 1.5% of cases, and respiratory failure in 0.7% of cases [56].
Factors influencing ENB diagnostic success include a larger nodule size (> 2 cm), upper or middle lobe location, and presence of a bronchial sign leading to the lesion [52]. Combining ENB with R-EBUS, utilizing rapid on-site cytological evaluation, and performing the procedure under general anesthesia have been shown to enhance accuracy.
Robotic bronchoscopy
Robot-assisted bronchoscopy enhances lung biopsy procedures by improving stability and maneuverability compared with conventional techniques. This method uses a robotic arm to guide a flexible tube equipped with a camera and biopsy instrument into the lungs (Table 6).
Monarch Platform (Auris Health Inc., Redwood City, CA, USA): Achieved 88.6% navigation success rate and 98.8% tissue acquisition rate, with a diagnostic yield of 69.1–77% [57]. The BENEFIT study reported a 96.2% lesion localization rate and 74.1% diagnostic yield [58].
Ion Lumen System (Intuitive Surgical, Sunnyvale, CA, USA): Uses shape-sensing technology, with a 98.7% navigation success rate and 81.7% diagnostic yield [59]. The PRECIsE study reported an overall diagnostic yield of 8% (82% for nodules ≤ 2 cm and 85% for nodules > 2 cm), with no severe pneumothorax complications [60].
Galaxy System (Noah Medical, San Carlos, CA, USA): An ongoing clinical trial (NCT06056128) is evaluating the accuracy of the TiLT+ technology in the Galaxy System™. Preliminary results from 15 peripheral pulmonary lesions (mean size: 20.5 mm) showed a 100% target reach, 86–93% diagnostic yield, and 3 reported complications [61].
Although robotic bronchoscopy offers enhanced precision and ease of navigation to peripheral nodules, its limitations include high cost and the requirement for general anesthesia. Future advancements and research will further define its role in clinical practice.
Bronchoscopic transparenchymal nodule access
It is a novel technique designed to detect pulmonary nodules lacking a direct airway path. Using CT, the bronchoscope is guided to a predetermined entry point, followed by needle access to the lung parenchyma and balloon dilation to facilitate sheath biopsy.
Fontaine-Delaruelle et al. [32] reported an 83% diagnostic yield with no major complications. The University of Heidelberg study demonstrated successful tract creation in five of six patients with previously inaccessible small nodules, with successful biopsies obtained [62]. However, two patients experienced pneumothorax, one of whom required intervention.
Surgical biopsy or resection
Surgical excisional biopsy remains the gold standard for the diagnosis and confirmation of pulmonary nodules. This method not only facilitates malignancy detection, but can also serve as a therapeutic approach in certain cases. In wedge resection performed via video-assisted thoracic surgery, intraoperative frozen section analysis is used to determine malignancy. If NSCLC is confirmed, immediate conversion to lobectomy or segmentectomy enables diagnosis, staging, and treatment in a single operation. For patients with clinical stage IA NSCLC (tumor size ≤ 2 cm, tumor-to-mass ratio > 0.5) and peripheral lesions, sublobar resection (segmentectomy or wedge resection) is increasingly preferred over lobectomy. Studies, including prospective nonrandomized trials and meta-analyses, suggest that sublobar resection offers favorable long-term survival for peripheral N0 lung cancers ≤ 2 cm [63,64]. However, frozen section analysis may be less reliable for small lesions (≤ 1.1 cm) or pre-malignant or early-stage pathological findings such as minimally invasive adenocarcinoma, adenocarcinoma in situ, or atypical adenomatous hyperplasia. In such instances, if NSCLC is later confirmed based on the final pathological results, complete lobectomy may still be necessary.
Diagnostic wedge resection using video-assisted thoracic surgery is particularly recommended for patients with an intermediate-to-high risk of malignancy when non-surgical biopsy results are inconclusive or suggest a malignancy [65,66]. This approach is particularly effective for nodules near the pleural surface, as it allows direct visual identification during surgery. For nodules located in deeper lung tissues, preoperative localization techniques [67–70], such as hook wire placement, fiducial markers, microcoils, or percutaneous methylene blue injection, can enhance the accuracy. Intraoperative imaging techniques, including technetium-99 radiation guidance, ultrasound, and fluoroscopy, can further improve nodule detection and resection precision.
CONCLUSION
Management of incidental pulmonary nodules remains a critical aspect of lung cancer screening and early detection. With the increasing prevalence of pulmonary nodules detected using LDCT screening, a structured, evidence-based approach is essential for accurately differentiating between benign and malignant nodules. Various risk stratification models, advanced imaging techniques, and biopsy methods collectively guide clinical decision-making and optimize patient outcomes, while minimizing unnecessary interventions.
Recent advancements in diagnostic tools, including PET scans, bronchoscopic techniques, and robot-assisted procedures, have enhanced the accuracy and safety of nodule evaluation. However, no single diagnostic modality provides a definitive solution, highlighting the importance of a multidisciplinary approach that integrates patient-specific risk factors, imaging characteristics, and clinical guidelines. Future research and clinical practice should focus on advancing risk assessment models and refining diagnostic tools to optimize the management of pulmonary nodules and ultimately improve lung cancer survival rates.
Notes
CRedit authorship contributions
SeungYong Park: conceptualization, resources, investigation, writing - original draft, project administration, funding acquisition; Seoung Ju Park: conceptualization, resources, writing - review & editing, supervision, project administration
Conflicts of interest
The authors disclose no conflicts.
Funding
This study was supported by funds from the Biomedical Research Institute, Jeonbuk National University Hospital.