Appropriate timing of antibiotic initiation in patients with sepsis or septic shock: a systematic review and meta-analysis
Article information
Abstract
Evidence supporting antibiotic administration within 3 hours in sepsis without shock is limited. Therefore, we conducted a systematic review and meta-analysis to determine whether the timing of antibiotic initiation influences mortality in patients with sepsis or septic shock. We comprehensively searched PubMed, EMBASE, the Cochrane Central Register of Controlled Trials, and the Korean Medical Database from inception to November, 2022, using the keywords “sepsis,” “septic shock,” “anti-bacterial agents,” “time to treatment,” and “time factors.” Two reviewers independently performed eligibility screening and full-text review. Thirteen studies including 79,246 patients were analyzed: five prospective, seven retrospective, and one retrospective case–control study. In overall sepsis cases, mortality did not differ significantly between patients who received antibiotics within 1 hour and those in the delayed group but was significantly lower in those who received antibiotics within 3 hours than in those in the delayed group. In patients with septic shock, mortality was significantly lower in groups that received antibiotics within both 1 and 3 hours than in the delayed group. In septic shock, administration of antibiotics within 1 hour of diagnosis reduces mortality. In patients with sepsis, antibiotic administration within 3 hours, but not necessarily within 1 hour, was associated with reduced mortality.
INTRODUCTION
Early administration of appropriate antibiotics is one of the most effective treatments for reducing mortality risk in patients with sepsis [1–3]. The 2016 Surviving Sepsis Campaign (SSC) guidelines recommend that patients with sepsis and septic shock receive antibiotics within 1 hour of emergency department triage [4]. Most studies on which these guidelines are based are retrospective observational studies, primarily involving patients admitted to the intensive care unit. Kumar et al. [5] reported a 7.6% increase in in-hospital mortality for each hour of delay in the administration of effective antibiotics after septic shock onset.
However, the optimal timing of antibiotic administration in sepsis remains controversial [6,7]. The Infectious Diseases Society of America (IDSA) does not support the 2016 SSC guidelines due to concerns that they could encourage antibiotic misuse and overdiagnosis of sepsis [8]. They noted the lack of evidence supporting early antibiotic initiation in patients with suspected sepsis without shock and the complexity of the definition of “time zero.” Therefore, they suggested that sepsis without shock should be excluded from bundled therapy, broad-spectrum antibiotics should be started within 1 hour of time zero for septic shock, and the definition of time zero should be clear and reproducible.
The SSC released a new version of its guidelines in 2021 [9]. The guidelines divide the time to antibiotic initiation based on the presence of shock and likelihood of infection, recommending that antibiotic administration be started within 1 hour in cases of septic shock and sepsis with a high likelihood of infection. In cases of possible sepsis without shock, a rapid investigation of the possibility of infection should be conducted, and antibiotics should be administered within 3 hours if concerns persist. The time-zero point for antibiotic administration was defined as the time of first recognition of sepsis.
Despite these changes, the quality of evidence for this recommendation remains poor in patients with sepsis but without shock. Previous studies have failed to differentiate between sepsis and septic shock and have used mixed criteria for time zero [10–13]. Therefore, we conducted a meta-analysis to determine whether the time from sepsis recognition to antibiotic initiation influences mortality in patients with and without septic shock.
METHODS
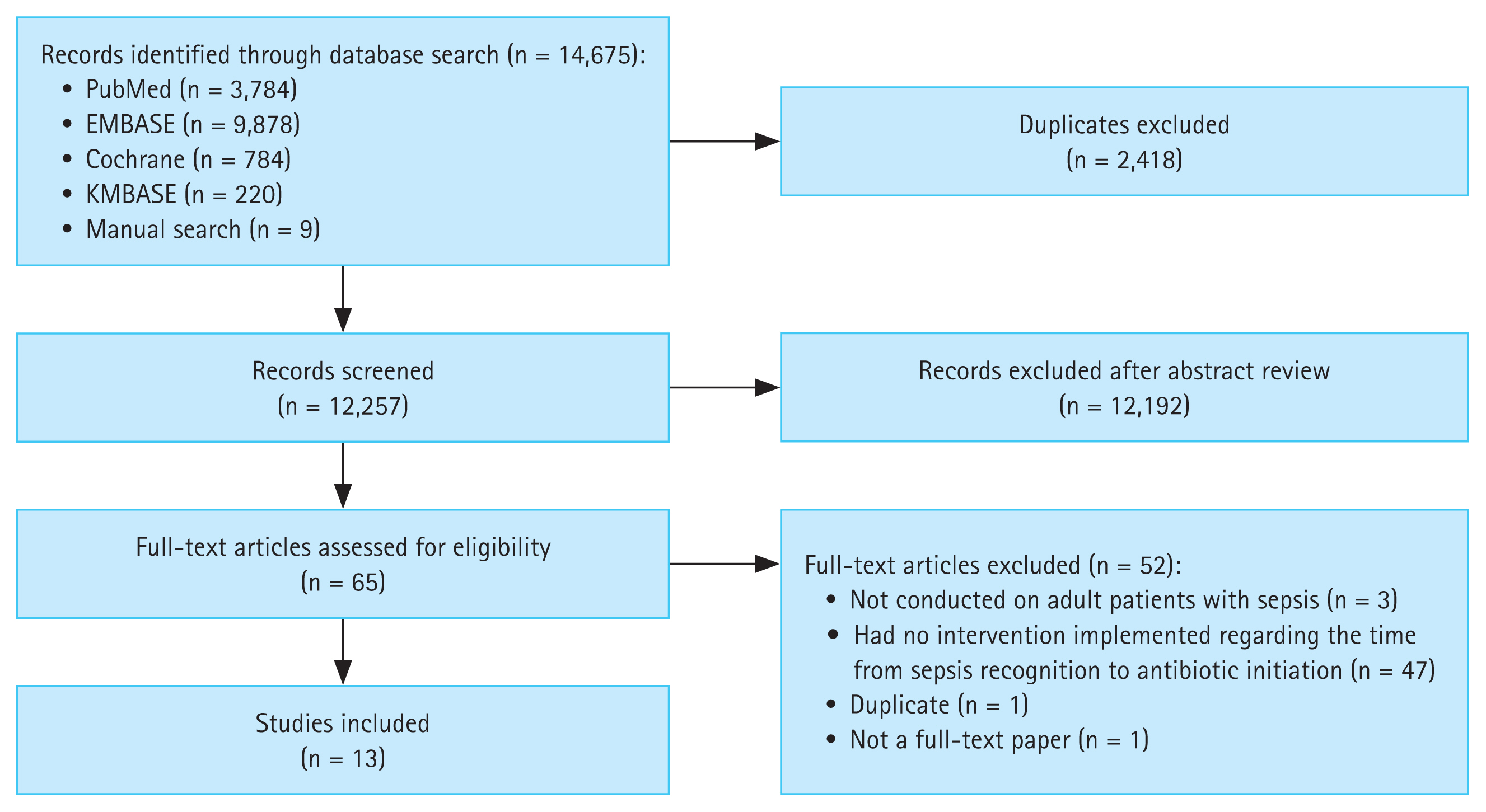
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [14] (Fig. 1). Approval from a specific Institutional Review Board or Ethics Committee was not sought because the study used publicly available data obtained from an online database. The study protocol is registered in PROSPERO (ID: CRD42024497641).
Search strategy
We performed a comprehensive search of PubMed, EMBASE, the Cochrane Central Register of Controlled Trials, and the Korean Medical Database from inception to November 22, 2022, to identify randomized controlled trials and cohort and case–control studies that assessed antibiotic initiation in sepsis. We restricted our search to clinical studies conducted on adult populations and published in English or Korean. The main search keywords were “sepsis,” “septic shock,” “anti-bacterial agents,” “time to treatment,” and “time factors” (Supplementary Table 1). The bibliographies of the retrieved publications and references of relevant reviews were also screened to ensure that no relevant studies were inadvertently omitted.
Study selection
Two reviewers (DP and NK) independently performed the initial eligibility screening of all retrieved titles and abstracts. Studies that reported original data specifically mentioning the time to antibiotic initiation in patients with sepsis or septic shock were selected for further review. Full-text reviews were independently performed by the same two reviewers using the following eligibility criteria: 1) randomized controlled trial, observational cohorts, and/or case–control studies; 2) inclusion of adult patients with sepsis; 3) description of antibiotic initiation timing; 4) establishment of a time-zero point for antibiotic initiation as the time of sepsis recognition; and 5) description of mortality outcomes. Any discrepancies were resolved by consensus discussion mediated by a third reviewer (YL). Unlike previous meta-analyses, our study included only those studies that defined time-zero for antibiotic administration as the time of sepsis recognition. Consequently, several studies that used alternative definitions of time zero (e.g., emergency department triage) were excluded, which may explain the smaller number of studies included in our analysis.
Assessment of risk of bias
The Risk of Bias Assessment Tool for Non-randomized Studies (RoBANS) (version 2) [15] was used to assess the quality of each included study based on the risk of bias across eight domains: comparability of the target group, target group selection, confounders, measurement of intervention/exposure, blinding of assessors, outcome assessment, incomplete outcome data, and selective outcome reporting. The risk of bias for each of these eight domains was rated as “low,” “high,” or “unclear.” Two reviewers (DP and NK) independently rated the quality of three articles using the RoBANS (version 2), and any disagreements were resolved through discussion and/or consultation with a third reviewer (YL). All three reviewers assessed the quality of the remaining articles.
Data extraction and statistical analysis
Data on the author, publication year, study type, study location, study population, severity of sepsis, time to antibiotic initiation, and mortality outcomes were extracted from each included study. RevMan software (version 5.4; The Cochrane Collaboration, 2020) and Comprehensive Meta-Analysis software (version 4; Biostat, Englewood, NJ, USA, 2022) were used to perform all statistical analyses and generate forest plots. A meta-analysis was performed only when at least two studies provided data on each outcome of interest. We extracted the adjusted odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) from the identified studies and used the inverse variance method to calculate the overall effect size with 95% CIs. If a study reported multiple adjusted ORs for the same outcome measure, a pooled effect size was calculated to represent the study outcome. Pooled estimates of ORs with 95% CIs were calculated using random- or fixed-effects models, depending on the degree of heterogeneity among the studies [16]. Statistical heterogeneity was assessed using I2 statistics, where values of 25%, 50%, and 75% were considered the cutoff points for low, moderate, and high degrees of heterogeneity, respectively [17]. A p value < 0.05 was considered statistically significant. Publication bias was assessed using the Egger’s regression model and visualized using a contour-enhanced funnel plot [18].
RESULTS
A total of 14,670 articles were initially retrieved. After duplicates were excluded, 12,257 articles remained. DP and NK screened titles and abstracts and excluded 12,192 irrelevant studies; the remaining 65 articles underwent full-text assessment. Fifty-two articles were excluded for the following reasons: three were not conducted on adult patients with sepsis, 47 did not implement any intervention on the time from sepsis recognition to antibiotic initiation, and two were either duplicates or not full-text papers. Thus, 13 articles were included in the final analysis (Table 1 and Fig. 1).
Study characteristics
Thirteen studies involving 79,246 patients were included in this meta-analysis: five prospective, seven retrospective; and one retrospective case–control (Table 1).
Effect of antibiotic administration within 1 hour
Ten of the 13 included studies analyzed the effects of antibiotic administration within 1 hour of sepsis and septic shock (overall sepsis). The mortality rate was 31.4% (28.9% [731/2,532] in the group administered antibiotics within 1 hour and 32.7% [1,632/4,993] in the group administered antibiotics after 1 hour). Pooled ORs from the 10 studies showed no significant differences in the mortality rate between the two groups (OR 0.87, 95% CI 0.75–1.01; p = 0.07], with moderate heterogeneity (I2 = 66%, p = 0.002; Fig. 2A).

Summary of forest plots. Pooled odds ratios for mortality and time to antibiotic administration within 1 hour or after 1 hour in patients with (A) overall sepsis and (B) septic shock. SE, standard error; IV, inverse variance; CI, confidence interval. Risk of bias legend: (A) Comparability of the target group; (B) Target group selection; (C) Confounders; (D) Measurement of intervention/exposure; (E) Blinding of assessors; (F) Outcome assessment; (G) Incomplete outcome data; (H) Selective outcome reporting.
The effect of antibiotic administration within 1 hour on mortality was investigated in six studies that included only patients with septic shock. The mortality rate was 28.6% (26.9% [189/702] in the group administered antibiotics within 1 hour and 29.2% [528/1,806] in the group administered antibiotics after 1 hour). Pooled ORs from the six studies showed the mortality rate was significantly lower in the group administered antibiotics within 1 hour than in the delayed group (OR 0.89, 95% CI 0.88–0.90; p < 0.001), with no significant heterogeneity (I2 = 15%, p = 0.32; Fig. 2B).
We performed a sensitivity analysis by excluding studies with different mortality endpoints. Liang et al. [24] used 90-day mortality as the outcome, whereas the other nine studies used either 28-day or in-hospital mortality (Table 1). In addition, the study by Liang et al. [24] included only older patients aged 65 years or older, which may have introduced heterogeneity in the analysis. After excluding this study, the pooled analysis results remained consistent with the original findings. Specifically, for patients with overall sepsis, no significant difference in mortality was observed between the group administered antibiotics within 1 hour and the delayed group (OR 0.86, 95% CI 0.73–1.01; p = 0.07), with moderate heterogeneity (I2 = 69%, p = 0.001; Supplementary Fig. 1A). In contrast, among patients with septic shock, the pooled analysis showed significantly lower mortality in the group administered antibiotics within 1 hour than in the delayed group (OR 0.83, 95% CI 0.71–0.98; p = 0.02), with no significant heterogeneity (I2 = 27%, p = 0.24; Supplementary Fig. 1B).
Effect of antibiotic administration within 3 hours
Seven of the 13 included studies analyzed the effect of antibiotic administration within 3 hours in patients with overall sepsis. The mortality rate was 32.7% (28.9% [862/2,981] in the group administered antibiotics within 3 hours and 39.1% [694/1,779] in the group administered antibiotics after 3 hours). Pooled ORs of the seven studies showed that the mortality rate was significantly lower in the group administered antibiotics within 3 hours than in the delayed group (OR 0.67, 95% CI 0.53–0.86; p = 0.001), with moderate heterogeneity (I2 = 63%, p = 0.01; Fig. 3A).

Summary of forest plots. Pooled odds ratios for mortality and time to antibiotic administration within 3 hours or after 3 hours in patients with (A) overall sepsis and (B) septic shock. SE, standard error; IV, inverse variance; CI, confidence interval. Risk of bias legend: (A) Comparability of the target group; (B) Target group selection; (C) Confounders; (D) Measurement of intervention/exposure; (E) Blinding of assessors; (F) Outcome assessment; (G) Incomplete outcome data; (H) Selective outcome reporting.
The effect of antibiotic administration within 3 hours on the mortality risk was investigated in two studies that included only patients with septic shock. The mortality rate was 25.6% (22.1% [205/929] in the group administered antibiotics within 3 hours and 31.7% [174/549] in the group administered antibiotics after 3 hours). Pooled ORs from the two studies showed that the mortality rate was significantly lower in the group administered antibiotics within 3 hours than in the delayed group (OR 0.65, 95% CI 0.51–0.83; p < 0.001), with no significant heterogeneity (I2 = 6%, p = 0.30; Fig. 3B).
Quality appraisal of included studies
Most of the included studies had a low risk of bias in all domains (Fig. 2, 3). Publication bias was assessed using funnel plots of standard error versus logit effect size. Examination of the funnel plots did not suggest publication bias, as no asymmetry was observed. Additional Egger regression tests revealed no significant publication bias in the included studies (Supplementary Fig. 2).
DISCUSSION
In this meta-analysis, antibiotic administration within 1 hour of sepsis recognition improved mortality in patients with septic shock but not in those without septic shock. Antibiotic administration within 3 hours improved mortality in both septic shock and overall sepsis cases. Thus, in patients with sepsis without septic shock, administering antibiotics within 1 hour did not have a significant effect on mortality, whereas administering antibiotics within 3 hours was found to be reasonable.
The SSC guidelines recommend that the likelihood of infection should be assessed first in patients with possible sepsis without shock and that antibiotics should be administered within 3 hours if concerns of infection persist after assessment [9]. Despite this recommendation, evidence supporting the administration of antibiotics within 3 hours in sepsis without shock has been limited. Therefore, in real-world clinical practice, concerns remain regarding delays in antibiotic administration related to the initial assessment of infection likelihood. This study showed that the administration of antibiotics within 1 hour had a mortality benefit in septic shock; however, it had no significant effect on mortality in sepsis without shock. These findings are important because they provide evidence to support the recommendations of the SSC guidelines.
Sepsis is difficult to distinguish from non-infectious syndromes. Up to 40% of patients admitted to intensive care units with a diagnosis of sepsis do not have an infection and thus do not actually have sepsis [30]. Assessing whether patients with organ dysfunction have an infection within a limited time can increase the risk of error. The increased risk of mortality associated with delayed antibiotic administration may prompt unnecessary antibiotic use even in cases with a minimal possibility of sepsis. Broad-spectrum antibiotics are commonly administered to patients with suspected sepsis. Rhee et al. [31] reported that although resistant pathogens were confirmed in only 29.2% of patients with sepsis, broad-spectrum empirical antibiotics targeting resistant organisms were administered to 67.7% of these patients. These findings highlight serious concerns regarding the unnecessary use of antibiotics in patients with suspected sepsis. The IDSA also raised this concern and suggested that in patients with uncertain infections and without shock, antibiotic administration should be postponed until additional diagnostic data are gathered to generate a more informed and precise therapeutic plan [8].
A meta-analysis published in 2015 also investigated the effect of time to antibiotic initiation on mortality risk in patients with severe sepsis or septic shock [10]. No significant benefit was observed from administering antibiotics within 1 hour of sepsis diagnosis. However, this study did not distinguish between sepsis with and without septic shock, making it difficult to determine whether there was a survival benefit from antibiotic use in patients with septic shock. Five of the eight studies included in the previous meta-analysis were also included in the current meta-analysis. The full texts of the three excluded studies were also reviewed but excluded because their time-zero point did not meet our criteria; they set emergency triage arrival as time zero. Eight additional studies published later were included in our analysis, totaling 13 studies.
A meta-analysis of 13 studies by Rothrock et al. [11] in 2020 showed no mortality benefit for patients who received antibiotics within 1 hour compared with those treated within 1–3 hours. In particular, a subgroup analysis of severe sepsis without shock found that antibiotics administered within 1 hour were associated with higher mortality than those administered within 1–3 hours. This finding is consistent with our results that antibiotic administration within 1 hour does not have a significant effect on mortality in patients with sepsis without shock. However, this analysis by Rothrock et al. [11] included studies with various time-zero points such as emergency triage arrival, onset of organ dysfunction, and onset of hypotension.
In another study by Huang et al. [12] in 2023, each hour delay in antibiotic administration was associated with an increased risk of mortality in adult patients with sepsis. However, the authors did not differentiate between sepsis cases with and without shock and did not adequately address time zero.
A recent meta-analysis by Tang et al. [13] investigated the effect of delayed antibiotic treatment on mortality in patients with sepsis or septic shock and found that antibiotic administration beyond 1 hour after emergency triage or sepsis identification was associated with increased mortality and antibiotic use within 3 hours was associated with decreased mortality. The effect of antibiotic administration within 3 hours was consistent with our results, showing reduced mortality in both septic shock and overall sepsis. However, this study also failed to differentiate between sepsis and septic shock and used mixed criteria for time zero.
We were unable to perform a meta-analysis exclusively for sepsis without shock, and instead analyzed the effect of time to antibiotic use in septic shock and overall sepsis to indirectly estimate its effect in sepsis without shock. Pak et al. [32] reported that delaying antibiotic administration for up to 6 hours from time zero (arrival at the emergency department) did not significantly increase mortality in patients with septic shock. In septic shock, the survival benefit of antibiotic administration within 1 hour was significant; however, in sepsis without shock, a significant increase in mortality risk was not observed until the time interval exceeded 9 hours (reference: within 1 hour). These results are consistent with the findings of our study and provide further evidence that delayed antibiotic initiation may be reasonable to adequately evaluate patients with sepsis without shock.
We found no effect of antibiotic initiation within 1 hour on mortality risk in patients with sepsis, consistent with the results of previous meta-analyses; however, early antibiotic administration was significantly beneficial when limited to septic shock cases.
The strengths of this study include the consistent application of the time-zero point defined as the time of sepsis recognition, as proposed by the SSC guidelines, and the separate evaluation of the effect of time to antibiotic initiation in septic shock and overall sepsis cases [9]. These findings provide evidence to support the SSC guideline recommendation that antibiotic use can be delayed by up to 3 hours in cases of possible sepsis without shock.
Our study has some limitations. First, all included studies were non-randomized cohort or case–control studies. Second, substantial heterogeneity was observed among the studies. This heterogeneity was not evident in analyses that included only patients with septic shock but was evident in analyses that included patients with sepsis. Finally, the appropriateness of empirically administered antibiotics was not considered. Although antibiotics are ineffective against viral and fungal infections and other non-infectious inflammatory diseases, it was difficult to evaluate this factor in our meta-analysis because of the lack of data on microbiology and antibiotic susceptibility. However, in the management of patients with sepsis, the causative organism is often unavailable at the time of antibiotic initiation, and negative culture results account for a significant proportion of sepsis cases. Therefore, our results, which did not differentiate among appropriate antibiotics, are likely to be more representative of real-world clinical practice.
In conclusion, in patients with septic shock, administering antibiotics within 1 hour of sepsis diagnosis reduces mortality risk. In patients with overall sepsis, administering antibiotics within 3 hours, but not within 1 hour, reduces mortality risk. Thus, in patients with sepsis without shock, administering antibiotics within 1 hour did not have a significant effect on mortality risk, and administering antibiotics within 3 hours appears to be plausible.
Notes
CRedit authorship contributions
Nam Su Ku: conceptualization, methodology, investigation, data curation, formal analysis, writing - review & editing, visualization, supervision; Yongseop Lee: writing - original draft; Dae Won Park: conceptualization, methodology, investigation, data curation, formal analysis, writing - review & editing, supervision
Conflicts of interest
The authors disclose no conflicts.
Funding
This work was supported by the Research Program funded by the Korea Disease Control and Prevention Agency (fund code 2022-10-016) and the Korean Society of Critical Care Medicine.