Comprehensive analysis of patients with rheumatoid arthritis associated interstitial lung disease
Article information
Abstract
Background/Aims
To investigate the demographics, disease characteristics, and treatment modalities of patients with rheumatoid arthritis (RA) associated interstitial lung disease (ILD), focusing on ILD exacerbation and mortality.
Methods
This retrospective study included individuals aged ≥ 18 years diagnosed with RA-ILD at Ajou University Hospital from January 1999 to March 2022. Diagnosis was based on chest computed tomography (CT) scans; progression was monitored based on available follow-up pulmonary function tests (PFTs) and chest CTs. Logistic regression analysis identified factors associated with ILD progression and mortality.
Results
The study included participants with a mean age of 64.3 years, 48.3% of whom were male. Smoking status: 13.2% ex-smokers, 25.2% current smokers, 61.6% non-smokers. Mean RA and ILD duration were 134.0 and 87.5 months, respectively. Mean Disease Activity Score in 28 joints was 4.9. The usual interstitial pneumonia (UIP) pattern was seen in 60.3%. Baseline PFT showed a mean FVC of 81.9 L, diffusing capacity for carbon monoxide (DLco) of 58.7 mL/min/mm, and DLco corrected for alveolar volume of 83.4 mL/min. With a mean follow-up of 4 years, ILD progressed in 58.3% of patients, with a mortality rate of 21.2%. ILD progression and UIP pattern significantly influenced mortality. Methotrexate use did not impact progression or mortality.
Conclusions
RA-ILD patients showed diverse clinical profiles, with ILD duration and UIP pattern significantly affecting prognosis. Personalized management and vigilant monitoring are essential to improve outcomes for RA-ILD patients.
INTRODUCTION
Rheumatoid arthritis (RA) is a significant challenge in the spectrum of autoimmune diseases. It is characterized by chronic synovial inflammation, which if inadequately controlled, leads to progressive joint destruction and functional disability [1]. Although articular involvement remains the primary manifestation of RA, it is imperative to acknowledge the systemic nature of this disease; it can significantly affect various organ systems, including the lungs. Interstitial lung disease (ILD) is one of the most prevalent and clinically significant pulmonary manifestations associated with RA, contributing to increased morbidity and mortality among affected patients [2].
RA-associated ILD (RA-ILD) presents complex and multifaceted challenges in clinical practice. Despite significant advancements in our understanding of RA, the etiology of RA-ILD remains unclear, likely owing to the interplay between environmental and genetic factors [2]. Notable risk factors, such as age at disease onset, sex, and smoking status, contribute to a higher incidence of ILD in certain patient subgroups [3]. Consequently, the presence of ILD among patients with RA makes diagnosis and treatment particularly challenging, thus complicating overall disease management. ILD can manifest in various patterns, ranging from non-specific interstitial pneumonia (NSIP) to usual interstitial pneumonia (UIP), each with a distinct clinical presentation and prognosis [4]. Identifying specific ILD patterns among patients with RA is crucial for tailoring appropriate treatment strategies and predicting disease outcomes [2,3].
Although significant advancements have been made in RA treatments, patients concurrently diagnosed with RA-ILD often experience treatment limitations [5]. Understanding the characteristics of these patients, identifying factors associated with ILD, and determining specific treatments to delay ILD progression are crucial for improving patient outcomes [6]. To address this gap in our knowledge, we conducted a retrospective analysis of patients diagnosed with RA-ILD. Our study aimed to comprehensively analyze various aspects, including ILD patterns, clinical characteristics, treatment modalities, and prognostic factors, to provide insights into the optimal management of patients with RA-ILD.
METHODS
Study design and data collection
This single-center, retrospective study was approved by the Institutional Review Board of Ajou University Hospital (approval number: AJOUIRB-DB-2024-269). The requirement for informed consent was waived due to the retrospective nature of this study. We reviewed the medical charts of patients diagnosed with RA-ILD at the Department of Rheumatology, Ajou University Hospital, between January 1999 and March 2022. The diagnosis of RA was based on patients aged ≥ 18 years, who fulfilled the 1987 American College of Rheumatology (ACR) criteria or the 2010 ACR/European League Against Rheumatism classification criteria [7,8]. Patients with RA-ILD were identified using diagnostic codes. ILD was identified using the International Classification of Diseases-10 codes M05.1, J99.0, or J84.0. Among patients with these diagnostic codes, only those with computed tomography (CT) scans interpreted by experienced radiologists, who confirmed inflammation or scarring of the lung interstitium or parenchyma, were included in this study.
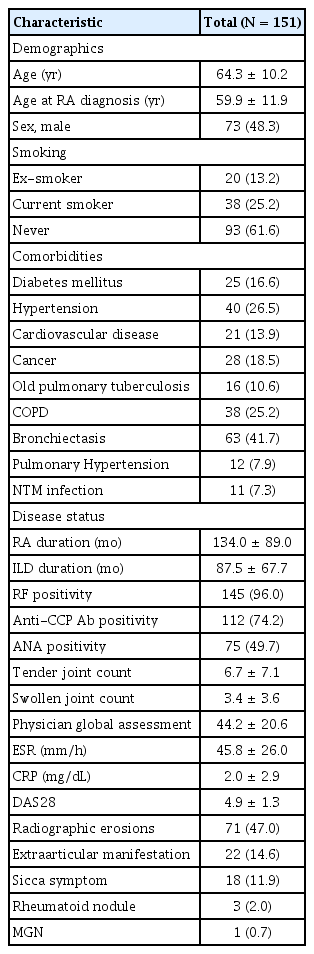
From their electronic medical records (EMRs), we gathered the patients’ comprehensive clinical data, including demographic information, comorbidities, RA disease characteristics, and pulmonary function test (PFT) results. We also documented their clinical characteristics, such as age, sex, body mass index (BMI), smoking habits, comorbid conditions, previous or current medications, erosions, and extra-articular manifestations. Laboratory findings included rheumatoid factor (RF), anti-cyclic citrullinated peptide antibody (anti-CCP Ab), C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR) levels. Additionally, we recorded the number of tender and swollen joints; pain visual analogue scale scores were extracted from the EMR. Using this information, we calculated the quantitative measures of RA disease activity, specifically the Disease Activity Score in 28 joints (DAS28) based on ESR and CRP levels.
Assessment of ILD via CT imaging and PFTs
CT scans, including traditional and high-resolution CT (HRCT), were evaluated by experienced radiologists who were blinded to the clinical data. ILD patterns were classified according to the American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Idiopathic Interstitial Pneumonia (2013), which included UIP, NSIP, lymphocytic interstitial pneumonia (LIP), organizing pneumonia (OP), and desquamative interstitial pneumonia (DIP) [9]. The PFTs included forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), diffusing capacity for carbon monoxide (DLco), and DLco corrected for alveolar volume (DLco/VA). The results were recorded for both volume (L) and percent predicted values.
ILD progression was monitored through PFTs; the extent of the lesions was observed on CT scans. ILD progression was defined by a relative decline FVC% predicted ≥ 10%, or DLco ≥ 15%, or an increased extent of fibrosis on CT scans. The extent of fibrosis on CT scans showing an increase of > 10% was also used as a criterion for ILD progression [9]. ILD stable was defined as a change in FVC % predicted and/ or DLco of less than 10%, with no significant change in the extent of fibrosis on CT scans. ILD improved was defined as an increase in FVC % predicted and/or DLco by more than 10% compared to baseline values. As this was a retrospective study, the timing of follow-up imaging studies and PFTs varied among patients, and progression was determined based on the most recent available follow-up data, which included corresponding PFT and CT scan results.
Statistical analysis
The patients’ baseline demographics and clinical characteristics included in this study were described with numerical variables presented as mean ± standard deviation and median (Q1–Q3), while categorical variables were expressed as number (%). A comparison between the two groups based on the ILD progression status was conducted. Categorical variables were then analyzed using the chi-squared test or Fisher’s exact test, while continuous variables were assessed using the Student’s t-test or Mann–Whitney U test, as appropriate. To compare the differences between the baseline and follow-up PFTs, a paired t-test was performed. Cox proportional hazards regression was applied to analyze factors influencing ILD exacerbation and patient survival. Variables for the multivariable analysis were selected using a stepwise selection method. All statistical analyses were performed using IBM SPSS software (version 26; IBM Corp., Armonk, NY, USA), with significance level set at p < 0.05.
RESULTS
Patient characteristics
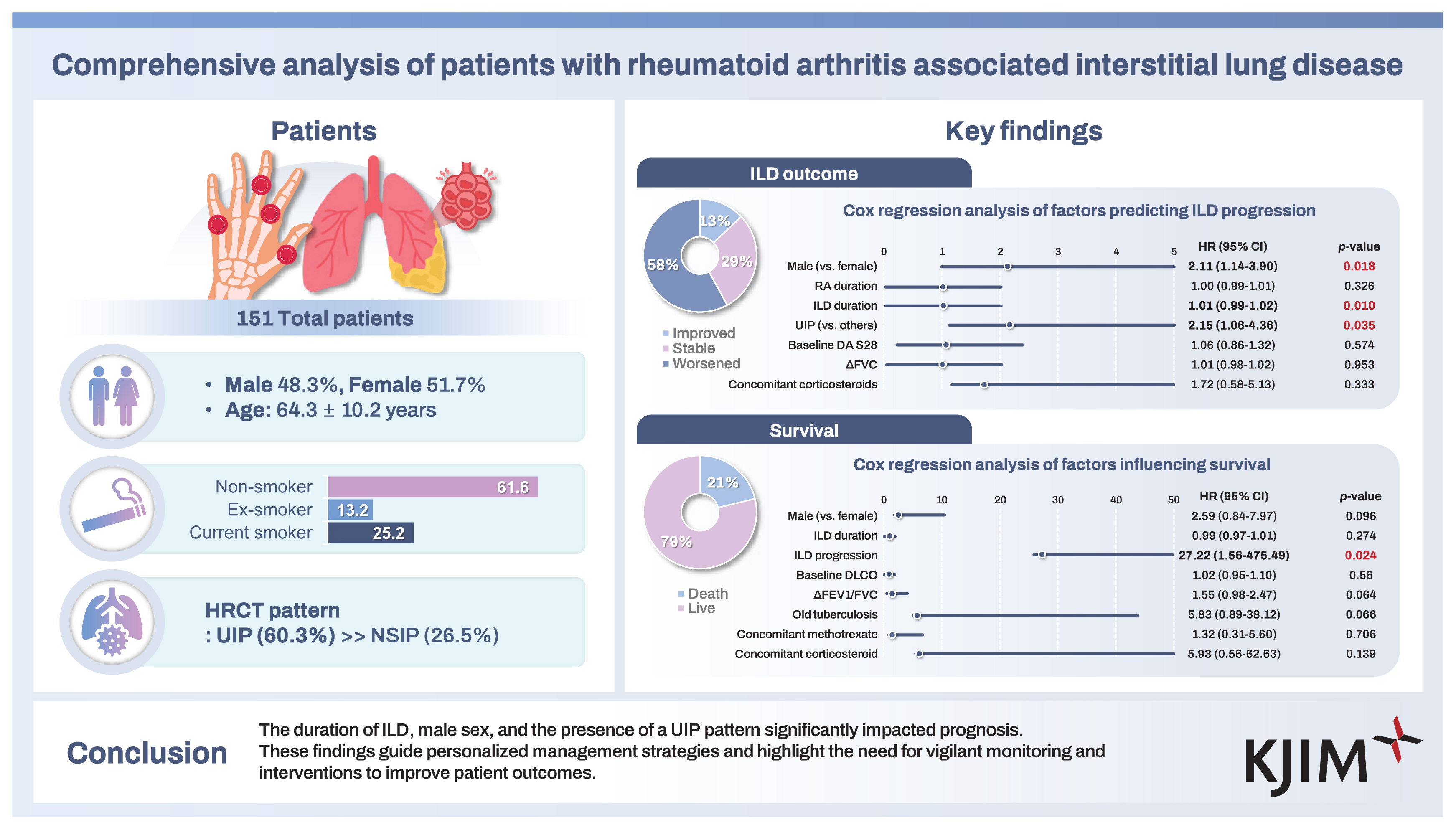
A total of 151 patients diagnosed with RA-ILD were included in this study. Their baseline characteristics are presented in Table 1. The mean age of the patients was 64.3 ± 10.2 years. The cohort was comprised of 73 males (48.3%) and of patients with variable smoking history, including 38 (25.2%) current smokers, 20 (13.2%) ex-smokers, and 93 (61.6%) non-smokers. Comorbidities included diabetes mellitus in 25 patients (16.6%), hypertension in 40 (26.5%), cardiovascular disease in 21 (13.9%), and cancer in 28 (18.5%). Regarding pulmonary conditions, 16 patients (10.6%) had old pulmonary tuberculosis, 38 (25.2%) had chronic obstructive pulmonary disease (COPD), 63 (41.7%) had bronchiectasis, 12 (7.9%) had pulmonary hypertension, and 11 (7.3%) had non-tuberculous mycobacterial infections. The mean durations of RA and ILD were 134.0 ± 89.0 months and 87.5 ± 67.7 months, respectively. RF-positive- and anti-CCP Ab-positive patients numbered 145 (96.0%) and 112 (74.2%), respectively, with a baseline DAS28 mean score of 4.9, indicating moderate disease activity. Radiographic erosions were observed in 71 patients (47.0%). Extra-articular manifestations included sicca symptoms in 18 patients (11.9%), rheumatoid nodules in 3 (2.0%), and membranous glomerulonephritis in 1 patient (0.7%).
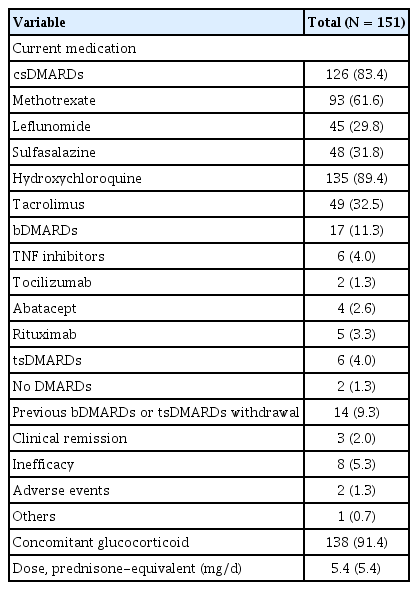
The medications used to treat RA in this study cohort are listed in Table 2. Out of the 151 patients, 126 (83.4%) were receiving conventional synthetic disease-modifying anti- rheumatic drugs (csDMARDs). Among them, hydroxychloroquine was the most commonly used, taken by 135 patients (89.4%). The other csDMARDs included methotrexate (93 patients, 61.6%), tacrolimus (49 patients, 32.5%), sulfasalazine (48 patients, 31.8%), and leflunomide (45 patients, 29.8%). Biological DMARDs (bDMARDs) were used in 17 patients (11.3%), with tumor necrosis factor inhibitors being the most common (6 patients, 4.0%), followed by rituximab (5 patients, 3.3%), abatacept (4 patients, 2.6%), and tocilizumab (2 patients, 1.3%). Targeted synthetic DMARDs (tsDMARDs) were used by 6 patients (4.0%). Additionally, a significant proportion of patients (138 patients, 91.4%) were on concomitant glucocorticoid therapy, with a mean prednisone-equivalent dose of 5.4 mg/d.
ILD patterns and PFT findings
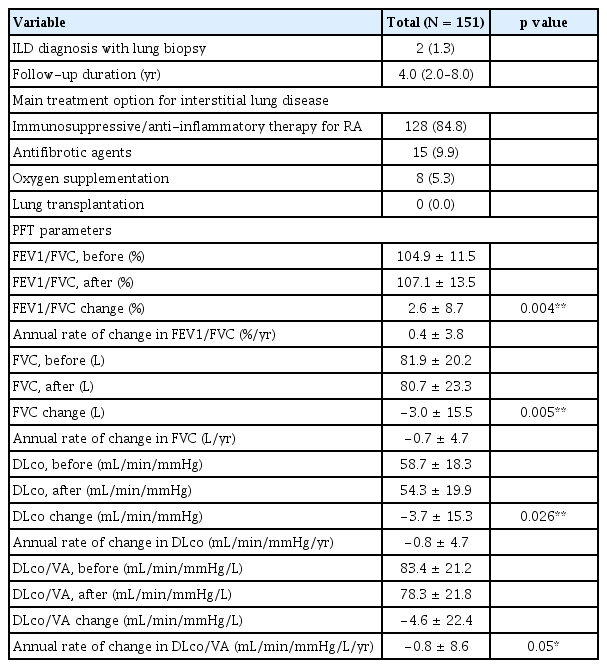
The CT patterns observed at the time of ILD diagnosis are shown in Figure 1 and ILD treatment modalities and PFT results are shown in Table 3. The CT patterns identified were as follows: UIP in 91 patients (60.2%); NSIP in 40 (26.5%); OP in in 9 (6.0%); DIP in 3 (2.0%); and unclassified in 8 patients (5.3%). Only 2 patients (1.3%) were diagnosed with ILD through lung biopsies. Immunosuppressive or anti-inflammatory therapy was the primary treatment choice for ILD in 128 patients (84.8%), followed by antifibrotic agents in 15 (9.9%) and oxygen supplementation in 8 patients (5.3%). None of the patients had previously undergone lung transplantation.

The patterns of chest HRCT. HRCT, high-resolution computed tomography; UIP, usual interstitial pneumonia; NSIP, non-specific interstitial pneumonia; LIP, lymphocytic interstitial pneumonia; OP, organizing pneumonia; DIP, desquamative interstitial pneumonia.
The median duration of patient follow-up was 4.0 years. In the baseline PFT parameters, the mean FEV1/FVC ratio measured 104.9 ± 11.5%, the mean FVC was 81.9 ± 20.2 L, the mean DLco was 58.7 ± 18.3 mL/min/mmHg, and the mean DLco/VA was 83.4 ± 21.2 mL/min/mmHg/L. The mean FEV1/FVC increased by 2.6% from baseline to follow-up, with a statistically significant difference (p = 0.004) and an annual rate of change of 0.4% increase per year. The changes in FVC and DLco were −3.0 L and −3.7 mL/min/mmHg, respectively, with annual rates of change of −0.7 L/yr and −0.8 mL/min/mmHg/yr, respectively; both also showed statistically significant differences from baseline to follow-up (p = 0.005 and p = 0.026, respectively). The change in DLco/VA was also significant, with a mean decrease of 4.6 mL/min/mmHg/L during the follow-up period and an annual rate of change of −0.8 mL/min/mmHg/L/yr (p = 0.05).
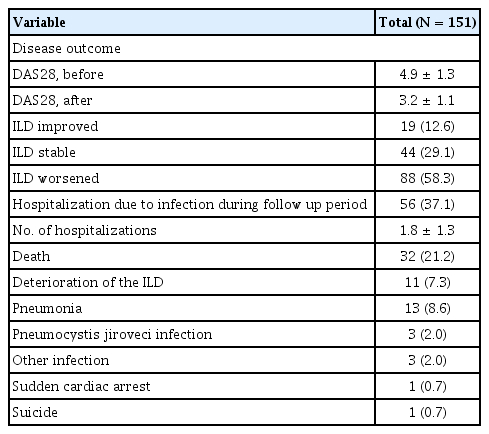
Patient outcomes in patients with RA-ILD
Table 4 shows various outcomes observed during the follow-up period of RA-ILD patients. The mean DAS28 score improved from 4.9 to 3.2. In terms of ILD progression, 88 patients (58.3%) experienced ILD progression, 44 patients (29.1%) were classified as ILD stable, and 19 patients (12.6%) as ILD improved. Among the patients, 56 (37.1%) were hospitalized because of infections, while 32 (21.2%) died; the majority of deaths were attributed to ILD exacerbation or pulmonary-related infections.
Analysis of factor influencing ILD progression and Survival
To identify factors associated with ILD progression, stepwise regression analysis was performed, selecting ILD duration, UIP pattern, changes in FVC, baseline DAS28, concomitant corticosteroid use, RA duration, and male sex as covariates. Cox regression analysis revealed that male sex (hazard ratio [HR] 2.11, 95% confidence interval [CI] 1.14–3.90, p = 0.018), ILD duration (HR 1.01, 95% CI 0.99–1.02, p = 0.010), and UIP pattern (HR 2.15, 95% CI 1.06–4.36, p = 0.035) were significant predictors of ILD progression (Fig. 2A). For overall survival, stepwise regression analysis selected male sex, ILD duration, ILD progression, baseline DLCO, changes in FEV1/FVC, old pulmonary tuberculosis, concomitant methotrexate use, and concomitant corticosteroid use as covariates. Figure 2B shows the results of the Cox regression analysis for overall survival, indicating that only ILD progression remained as a significant predictor of patient survival (HR 27.22, 95% CI 1.56–475.49, p = 0.024).

Cox regression analysis of factors influencing ILD progression and survival. (A) Factors predicting ILD progression in patients with RA with ILD. (B) Influence of ILD patterns and other variables on survival in patients with RA with ILD. ILD, interstitial lung disease; RA, rheumatoid arthritis; HR, hazard ratio; CI, confidence interval; UIP, usual interstitial pneumonia; DAS28, Disease Activity Score in 28 joints; FVC, forced vital capacity; DLco, diffusing capacity for carbon monoxide; FEV1, forced expiratory volume in 1 second.
DISCUSSION
This study was conducted at a single center with a long-term follow-up of patients with RA-ILD, which may provide valuable insights into the progression and outcomes of RA-ILD. Despite numerous studies on the prognosis and outcomes of concurrent RA-ILD, recent reports have indicated its increasing prevalence, but with no consensus on its risk and predictive factors; this highlights the ongoing importance of the current study [6,10]. The patients included in our study exhibited a higher age and proportion of males as compared to the general RA population, a characteristic consistent with findings observed in other RA-ILD cohorts [3,6,11]. Furthermore, while the treatment rates for bDMARDs and tsDMARDs were generally comparable, a slightly higher proportion of patients used glucocorticoids [12]. This contrasted with recent treatment trends advocating for glucocorticoid use only as a form of bridging therapy, possibly because of the association of DMARDs with pulmonary toxicity as well as the clinicians’ tendency to opt for glucocorticoids to mitigate the risk of ILD exacerbation [13].
In our study, the patient cohort manifested a restrictive pattern without airflow limitation, maintaining a relatively preserved pulmonary function. However, the observed decline in the FVC, DLco, and DLco/VA throughout the follow-up period underscored the progressive nature of pulmonary impairment among RA-ILD patients. These trends aligned with those of previous studies on the changes in pulmonary function among patients with RA-ILD [14]. However, the rate of ILD progression in RA-ILD varies significantly depending on the definition of disease progression, characteristics of the study population, and the duration of follow-up, thereby making direct comparisons challenging. We employed definitions commonly used in large-scale retrospective studies; accordingly, some reports have shown ILD progression rates ranging from 30–50% [15–17]. In this study, approximately 60% of patients showed ILD progression, slightly higher than those reported in other studies.
Several factors may have accounted for this discrepancy. Due to the longer data collection period as compared with other studies, our cohort included patients diagnosed many years ago; thus, they likely did not benefit from the current therapeutic effects of biological agents when ILD or RA worsened. Additionally, our study included only a small number of patients who used antifibrotic agents, thus reflecting the limited access to these newer treatments for RA-ILD in South Korea. Despite the proven efficacy of nintedanib and pirfenidone in treating RA-ILD, stringent insurance coverage criteria and high costs make it challenging to provide these medications to concerned patients [18,19]. Considering that the majority of fibrosing ILD patients with a progressive phenotype in South Korea have connective tissue disease-associated ILD and that there are increasing reports on the effectiveness and safety of antifibrotic agents in RA-ILD, the outlook for ILD treatment appears promising [20,21].
As crucial as advancements in ILD treatment for improving prognosis, identifying factors that exacerbate ILD progression as well as mitigating their impact is important. Numerous studies have extensively documented the risk factors that exacerbate the progression of RA-ILD, including older age, male sex, disease activity, reduced FVC, presence of a UIP pattern, and smoking history [22–27]. Our study corroborates these findings, emphasizing the significant impact of male sex, UIP patterns and ILD duration on disease progression. Unfortunately, the presence of a UIP pattern among RA-ILD patients is unalterable, unlike factors such as medication selection and smoking history. Specifically, the UIP pattern was more prevalent in RA patients, rather than in those with other autoimmune conditions [28]. Therefore, patients with RA require thorough screening via methods such as HRCT; appropriate treatment and regular monitoring are essential if ILD, particularly with a suspected UIP pattern, is detected. Emerging evidence suggests the potential efficacy of bDMARDs in delaying or ameliorating RA-ILD progression, with recent reports indicating the promising therapeutic effects of JAK inhibitors [29–31]. Thus, even in the absence of severe joint symptoms, consideration should be given when transitioning from bDMARDs or tsDMARDs, particularly if rapid ILD progression or respiratory deterioration is observed during clinical assessment or PF testing.
In the present study, we did not obtain significant results regarding the effects of medication on ILD progression. This somewhat differed from previous findings suggesting that methotrexate may prevent disease progression; other studies have indicated that leflunomide could exacerbate ILD [11,12]. Recent reports have suggested that methotrexate may not exert a significant effect on ILD. Since our study included patients diagnosed with RA and ILD many years ago, it is likely that methotrexate was not used in patients with severe ILD at that time, thus leading to inconclusive results. Additionally, the limited number of patients using bDMARDs, such as abatacept and tocilizumab, which have been proven effective in improving RA-ILD, may have contributed to the lack of statistical significance [32,33]. One interesting observation was the tendency of lower mortality rates among patients treated with leflunomide. Although leflunomide is generally avoided in patients with ILD owing to pulmonary toxicity concerns, its association with favorable outcomes in RA-ILD suggested that it may have a less detrimental effect on lung function in patients with early ILD and could potentially better regulate RA disease activity. This could be attributed to the relatively preserved baseline lung function of the patients included in this study, which indicated a high proportion of early ILD cases. Given similar findings in previous studies, reconsideration of the role of leflunomide in RA-ILD is warranted [23].
The continued emphasis on the importance of diagnosing and treating ILD in patients with RA stems from the fact that they not only have higher mortality rates than the overall RA population, but also because ILD exhibits the highest mortality rate among pulmonary complications associated with RA [34–36]. Therefore, we investigated the mortality factors in RA-ILD, and demonstrated that only ILD progression is a direct factor that increases the mortality rate. Unlike other reports suggesting that average glucocorticoid dose, age, and COPD history are relevant factors, our study emphasized the prioritization of ILD management for patients with RA-ILD [37]. Preventing ILD progression not only extends lifespan but also improves quality of life by preserving respiratory function, promoting physical activity, and fostering emotional stability throughout the lifetime [38].
This study had significant strengths in the longitudinal monitoring of patients with RA-ILD, providing valuable insights into their long-term characteristics. However, this study also had a few limitations. First, as a retrospective study, the varying follow-up durations inherent to its design make it challenging to establish a definition of ILD progression that aligns perfectly with that of a clinical trial. Another limitation of this study is its single-center design, which could introduce the possibility of selection bias and thus limiting the generalizability of the findings to broader populations. Additionally, the relatively low number of recently diagnosed patients, coupled with the characteristics of the Korean insurance system, notably limited the proportion of patients receiving treatment with antifibrotic agents and tsDMARDs.
Conclusions
This study provided a comprehensive exploration of patient demographics, treatment modalities, and clinical outcomes, thus offering profound insights into the multifaceted nature of RA-ILD. Our analysis revealed valuable findings regarding factors that influence disease progression and patient survival. Notably, a significant proportion of patients experienced ILD progression, with the UIP pattern and a prolonged ILD duration emerging as pivotal predictors of disease progression. Furthermore, our results emphasize the critical role of ILD progression as the primary determinant of patient mortality. These findings highlight the importance of early diagnosis in suspected RA-ILD cases to confirm the ILD pattern and emphasize the need for close monitoring and timely intervention for effective disease management. Additionally, personalized treatment approaches incorporating innovative modalities, including b/tsDMARDs or antifibrotic agents, remains crucial for preventing disease progression.
KEY MESSAGE
1. The UIP pattern and ILD duration significantly influence disease progression and mortality in RA-ILD patients.
2. Methotrexate use did not show a significant impact on the progression or mortality of RA-ILD in this cohort.
3. Personalized management and close monitoring are critical for improving outcomes in RA-ILD patients.
Notes
CRedit authorship contributions
Ji-Won Kim: conceptualization, methodology, resources, investigation, data curation, formal analysis, validation, software, writing - original draft; Ju-Yang Jung: methodology, resources, investigation, data curation, supervision; Chang-Hee Suh: methodology, resources, investigation, data curation, supervision; Hyoun-Ah Kim: conceptualization, methodology, resources, investigation, data curation, formal analysis, validation, software, writing - review & editing, visualization, supervision, project administration
Conflicts of interest
The authors disclose no conflicts.
Funding
None