Live zoster vaccination and hepatobiliary risk reduction: a nationwide South Korean study
Article information
Abstract
Background/Aims
Herpes zoster (HZ) vaccination is primarily administered to prevent shingles, yet its systemic immunomodulatory effects may offer protection against other organ-related diseases, including hepatobiliary and pancreatic diseases. Therefore, this emulated target trial aimed to evaluate whether live HZ vaccination reduces the long-term risk of hepatobiliary diseases in older adults.
Methods
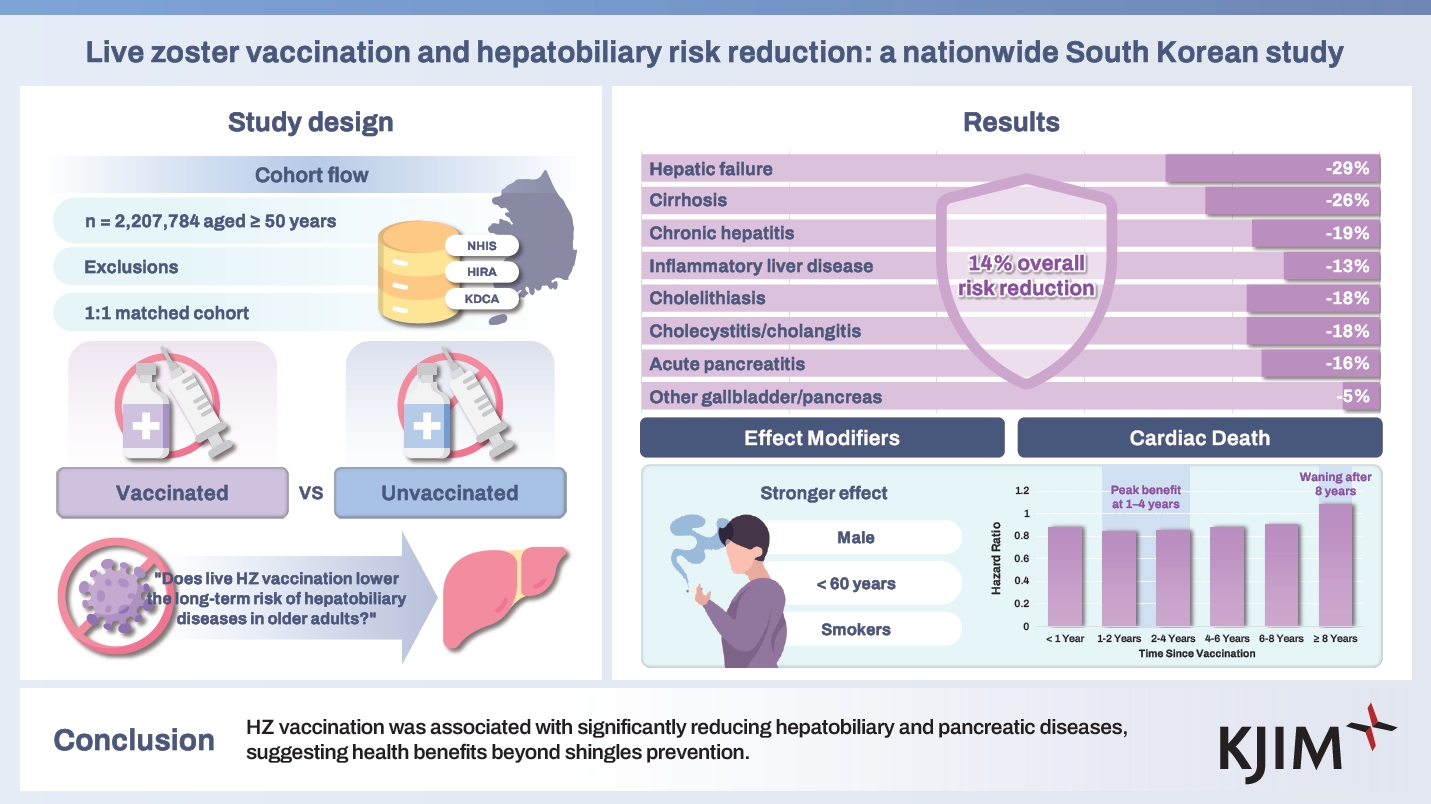
We conducted a nationwide, population-based cohort study in South Korea (n = 2,207,784 individuals aged ≥ 50 years) from January 1, 2012, to December 31, 2021, with follow-up until January 31, 2024. This cohort was built by merging health insurance (Korea Health Insurance Review and Assessment Service), national health screening (Korean National Health Insurance Service), and vaccination records (Korea Disease Control and Prevention Agency). To assess the risk of any hepatobiliary diseases and eight subcategories following HZ vaccination, we performed 1:1 exposure-driven propensity score matching and estimated adjusted hazard ratios (aHRs) using Cox proportional hazards models.
Results
After matching, 1,462,070 individuals were included (mean age, 61.57 years; 56.26% females). HZ vaccination was associated with a 14% lower risk of developing any hepatobiliary events (aHR 0.86, 95% CI 0.85–0.87). Risk reductions were consistent across all subcategories, notably for hepatic failure (aHR 0.71, 95% CI 0.63–0.78) and liver cirrhosis (aHR 0.74, 95% CI 0.70–0.77). Protective associations were more pronounced in males, younger individuals (< 60 years), and smokers. The benefit persisted for eight years, peaking within the first four years.
Conclusions
HZ vaccination was associated with significantly reducing hepatobiliary and pancreatic diseases, supporting potential broader health benefits beyond shingles prevention in older adults.
INTRODUCTION
Herpes zoster (HZ), or shingles, results from the reactivation of the latent varicella-zoster virus (VZV) and commonly affects among older adults [1]. Its lifetime incidence is approximately one-third, increasing markedly after the age of 50 years due to immunosenescence [1,2]. While HZ typically manifests as a painful vesicular rash, it may also lead to systemic and long-term complications. Well-known complications include postherpetic neuralgia [3], ophthalmic involvement [4], and neurological sequelae [5,6]. These complications suggest that HZ can affect various organ systems through systemic inflammation or direct viral injury.
Two vaccines are available to prevent HZ in adults aged ≥ 50 years: the live-attenuated zoster vaccine (Zostavax; Merck & Co., Inc., Rahway, NJ, USA) [6] and the recombinant zoster vaccine (Shingrix; GlaxoSmithKline, London, UK) [6-8] While the primary goal of vaccination is to diminish HZ incidence and severity, recent studies suggest broader benefits, including reduced dementia risk [9]. Given its potential systemic effects, vaccination may confer protection against other conditions, including hepatobiliary and pancreatic, though this remains understudied.
There is growing recognition that HZ infection may contribute to hepatobiliary dysfunction through direct viral effects or immune-mediated pathways. Some studies have documented VZV reactivation leading to acute hepatitis or fulminant hepatic failure, particularly in immunocompromised patients [10,11], and VZV infection has been implicated in cases of acute pancreatitis [12]. Furthermore, individuals with liver cirrhosis exhibit a higher risk of developing HZ than the general population [13], suggesting an interplay between liver dysfunction and VZV reactivation. These findings raise the question of whether preventing HZ through vaccination could also reduce the incidence of hepatobiliary diseases in the general population.
Despite these observations, no long-term, large-scale study has comprehensively investigated the association between HZ vaccination and hepatobiliary disease risk. Therefore, this study uses a nationwide, population-based cohort in South Korea, encompassing over two million participants with up to 12 years of follow-up, to assess the long-term risk reduction of developing hepatobiliary and pancreatic conditions following HZ vaccination.
METHODS
Data source
This is a large-scale, population-based cohort study in South Korea, comprising 2,207,784 individuals. The cohort is built upon the country’s universal health insurance system, integrating multiple national databases: outpatient and inpatient records, pharmaceutical data, and mortality records from the Korea Health Insurance Review and Assessment Service (HIRA); national health examination results from the Korean National Health Insurance Service (NHIS); and live zoster vaccination records from the Korea Disease Control and Prevention Agency (KDCA). The study protocol was approved by the Korea Healthcare Bigdata of the Ministry of Health and Welfare (No. 2022-00061), HIRA, NHIS, and KDCA. As per the approval terms, patient consent was waived due to the use of anonymized health records. All patient data were de-identified by the Korea Healthcare Bigdata of the Ministry of Health and Welfare to ensure confidentiality.
This cohort represents nearly 100% of the South Korean population [14,15] and includes all individuals aged ≥ 50 years who received the live zoster vaccine between January 1, 2012, and December 31, 2021. We excluded those who meet the following criteria: (1) insufficient socioeconomic information or death before enrollment; (2) missing national health examination data; and (3) a history of hepatobiliary events prior to HZ vaccination (n = 713,871 excluded). A 1:1 propensity score-matched control group was selected from unvaccinated individuals with similar demographic characteristics. After matching, the final sample consisted of 1,462,070 participants (731,035 vaccinated vs. 731,035 unvaccinated), with an observation period from January 1, 2012, to January 31, 2024. Details on the cohort selection are provided in Supplementary Methods.
Exposures and outcomes
In this study, HZ vaccination was defined as the exposure. A total of 1,093,860 individuals within this cohort received the HZ vaccine between January 1, 2012, and December 31, 2021. The primary outcome of interest was the new onset of any hepatobiliary events occurring at least 30 days post-vaccination to minimize reverse causation [16]. The secondary outcomes encompassed the following eight hepatobiliary events: (1) hepatic failure; (2) inflammatory liver disease; (3) chronic hepatitis; (4) liver cirrhosis; (5) cholelithiasis; (6) cholecystitis, cholangitis; (7) acute pancreatitis; and (8) other diseases of the gallbladder and the pancreas [17]. The follow-up period ended at the earliest of the following events: January 31, 2024, the onset of a hepatobiliary event, or death. A list of the International Classification of Diseases, 10th Revision (ICD-10) codes for each disease is provided in Supplementary Table 1 [17].
Covariates
Participant demographics were obtained from the national health insurance database and included age (50–54, 55–59, 60–64, ≥ 65 years), sex, residential area (urban vs. rural), and household income (low [0–39th percentile], middle [40–79th percentile], and high [80–100th percentile]). Clinical characteristics were derived from national health examination records and included body mass index (BMI; underweight/normal [< 23.0 kg/m2]; overweight [23.0–24.9 kg/m2], and obese [≥ 25.0 kg/m2]), blood pressure (normal [systolic < 140 mmHg and diastolic < 90 mmHg] and hypertensive [systolic ≥ 140 mmHg or diastolic ≥ 90 mmHg]), fasting blood glucose (< 100 mg/dL vs. ≥ 100 mg/dL), and glomerular filtration rate (< 60, 60–90, and ≥ 90 mL/min/1.73 m2) [14,15].
Comorbidity data were assessed using the Charlson comorbidity index (CCI), while medication history for hypertension, coronary artery disease, diabetes, and hyperlipidemia was identified through ICD-10 codes and prescription records. Lifestyle factors were also included: smoking status (non-smoker, former smoker, current smoker), alcohol consumption (< 1, 1–2, 3–4, and ≥ 5 days per week), and aerobic physical activity (≥ 600 metabolic equivalent task [MET] scores vs. < 600 MET scores) [18]. To ensure data completeness, health examination records were matched with the most recent available data up to or including the index date. In addition, a complete-case approach was applied, with individuals missing any covariate data excluded from the analysis.
Propensity score matching
We ensured balance in the distribution of covariates between the vaccinated and unvaccinated groups by implementing 1:1 exposure-driven propensity score matching. A greedy nearest-neighbor algorithm was used with a random selection without replacement within a 0.001 standard deviation caliper width [17]. Matching adequacy was evaluated using standardized mean differences (SMDs), with an SMD < 0.1 indicating no significant imbalance. The variables used for matching included age, sex, household income, residential area, and CCI. After propensity score matching, the final study population comprised 1,462,070 individuals (Fig. 1, Supplementary Table 2). To address immortal time bias, external comparators were assigned the same index date as their matched vaccinated counterparts.
Statistical analysis
To evaluate the association between HZ vaccination and the onset of hepatobiliary outcomes, Cox proportional hazard models were used to derive adjusted hazard ratios (aHRs) with 95% confidence intervals (CIs) [17]. We further performed stratification analysis by sex, age, residential area, household income, BMI, smoking status, alcohol consumption, physical activity, and CCI, as well as medication use for coronary artery disease, diabetes, hyperlipidemia, and hypertension. We also assessed the time-persistent effect of hepatobiliary events post-vaccination, categorizing follow-up into < 1, 1–2, 2–4, 4–6, 6–8, ≥ 8 years). Justifications for sensitivity analyses are provided in Supplementary Figure 1. All statistical analyses were conducted using SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA), with a two-sided p value < 0.05 considered statistically significant.
Patient and public involvement
Patients were not involved in defining the research question, study design, or implementation. However, we plan to share the findings with study participants and relevant communities upon request.
RESULTS
Baseline characteristics
A total of 2,207,784 individuals aged ≥ 50 years were included in the initial cohort (Table 1). The baseline characteristics for the full unmatched cohort are presented in Supplementary Table 3. Among these participants, 1,093,860 (49.5%) received an HZ vaccination during the study period. After applying exclusion criteria and performing 1:1 propensity score matching, 1,462,070 individuals were included in the matched cohort (731,035 vaccinated and 731,035 unvaccinated). The matching process achieved balance in baseline characteristics; all covariate SMDs were < 0.1, indicating no significant differences between the vaccinated and unvaccinated groups. The mean age of the matched cohort was 61.57 years (standard deviation, 3.47 years), and 56.26% (822,493) of the participants were female. Additional baseline characteristics, including health check-up records and lifestyle factors, are provided in Table 1.
Reduction in hepatobiliary disease risk following HZ vaccination
Figure 2 and Supplementary Table 4 illustrate the risk for overall and each hepatobiliary outcome following HZ vaccination. The incident risk of any hepatobiliary events was 14% lower in vaccinated individuals than in unvaccinated individuals (aHR 0.86, 95% CI 0.85–0.87). Specifically, HZ vaccination was associated with a 29% lower risk of hepatic failure (aHR 0.71, 95% CI 0.63–0.78), which was the most pronounced reduction among the outcomes studied. Likewise, the vaccinated group showed lower incident risks of liver cirrhosis (aHR 0.74, 95% CI 0.70–0.77), chronic hepatitis (aHR 0.81, 95% CI 0.80–0.83), and inflammatory liver disease (aHR 0.87, 95% CI 0.86–0.88). For biliary and pancreatic diseases, compared to individuals who did not receive HZ vaccination, vaccinated individuals showed significantly lower risks of cholelithiasis (aHR 0.82, 95% CI 0.80–0.84), cholecystitis/cholangitis (aHR 0.82, 95% CI 0.80–0.85), acute pancreatitis (aHR 0.84, 95% CI 0.80–0.88), and other diseases of the gallbladder and pancreas (aHR 0.95, 95% CI 0.93–0.96).
Stratified analyses
Figure 3 and Supplementary Table 5 show that according to the stratification analysis, males had a more pronounced reduction in any hepatobiliary events compared to females (males: aHR 0.83, 95% CI 0.81–0.84; females: aHR 0.88, 95% CI 0.87–0.89). Individuals aged < 60 years exhibited a lower risk (aHR 0.79, 95% CI 0.78–0.80) compared to those aged ≥ 60 (aHR 0.90, 95% CI 0.89–0.91), while individuals with a smoking history exhibited a greater risk reduction (aHR 0.83, 95% CI 0.82–0.84) than never-smokers (aHR 0.87, 95% CI 0.86–0.88) for any hepatobiliary events. When stratified by alcohol consumption, individuals who drink alcohol 1–4 days per week had the least risk reduction (aHR 0.90, 95% CI 0.89–0.91) than those who drink alcohol < 1 day (aHR 0.80, 95% CI 0.79–0.81) and ≥ 5 days per week (aHR 0.81, 95% CI 0.76–0.86). When stratified by household income, those with low socioeconomic status have a slightly stronger association (low: aHR 0.83, 95% CI 0.82–0.85; middle: aHR 0.86, 95% CI 0.85–0.88; high: aHR 0.87, 95% CI 0.86–0.89).

Stratification analysis of the incident risk of hepatobiliary diseases following zoster vaccination in the propensity score-matched cohort. aHR, adjusted hazard ratio; CI, confidence interval. a)Others include other diseases of the gallbladder and pancreas. b)Non-smoker indicates individuals who do not currently smoke. c)Medication use.
The risk reduction was similar across the regions of residence (urban: aHR 0.87, 95% CI 0.86–0.88; rural: aHR 0.85, 95% CI 0.84–0.86), BMI (< 23.0 kg/m 2 : aHR 0.88, 95% CI 0.86–0.89; 23.0–24.9 kg/m 2 : aHR 0.85, 95% CI 0.84–0.87; ≥ 25.0 kg/m 2 : aHR 0.85, 95% CI 0.84–0.86), physical activity (insufficient: aHR 0.86, 95% CI 0.85–0.87; sufficient: aHR 0.86, 95% CI 0.84–0.87), medication use for coronary artery disease (no: aHR 0.86, 95% CI 0.85–0.87; yes: aHR 0.88, 95% CI 0.84–0.92), medication use for diabetes (no: aHR 0.86, 95% CI 0.85–0.87; yes: aHR 0.84, 95% CI 0.82–0.86), medication use for hyperlipidemia (no: aHR 0.86, 95% CI 0.85–0.87; yes: aHR 0.83, 95% CI 0.81–0.85), and medication use for hypertension (no: aHR 0.86, 95% CI 0.85–0.87; yes: aHR 0.85, 95% CI 0.84–0.86). Similar results were found across eight hepatobiliary outcomes (Supplementary Tables 6-13).
Time-dependent effects
The association between HZ vaccination and the risk of hepatobiliary diseases across different post-vaccination time intervals (< 1, 1–2, 2–4, 4–6, 6–8, and ≥ 8 years) was assessed, as presented in Figure 4. There was a significant protective effect within the first year after vaccination (aHR 0.88, 95% CI 0.86–0.89), which became more pronounced between 1 and 4 years (1–2 years: aHR 0.84, 95% CI 0.83–0.86; 2–4 years: aHR 0.85, 95% CI 0.83–0.86). The association slightly attenuated between 4 and 6 years (aHR 0.88, 95% CI 0.86–0.90) and further declined at 6–8 years (aHR 0.90, 95% CI 0.86–0.94). After eight years, no significant protective association was observed (aHR 1.08, 95% CI 0.84–1.39). These findings were consistent across eight hepatobiliary outcomes (Supplementary Tables 14-22), with the protective association generally persisting for up to eight years post-vaccination, though variations were observed within this period.
DISCUSSION
Principal findings of this study
This large, nationwide, population-based cohort study, encompassing over a million individuals in South Korea, is the first to assess the association between live HZ vaccination and a reduced risk of hepatobiliary diseases. First, vaccinated individuals had a 5–29% lower risk of developing any hepatobiliary events. Second, the risk reduction was most pronounced for hepatic failure and cirrhosis—outcomes with high morbidity and mortality—suggesting that preventing HZ might be particularly beneficial in averting severe liver decompensation events. Even for more common chronic conditions, such as chronic hepatitis or gallstone disease, the vaccine significantly reduced the risk of incident cases. Third, with a long-term follow-up of up to 12 years, this protective association persists for eight years, with the greatest risk reduction occurring in one to four years for most diseases, then gradually attenuating, which is consistent with the known duration of immunity following the live HZ vaccine. Fourth, these findings remained robust across multiple subgroup analyses, with the protective association observed across different age, sex, socioeconomic, and health behavior groups. However, the magnitude of the association varied, with stronger benefits seen in males, younger individuals (< 50 years), and those with unhealthy lifestyles. Overall, our findings support the hypothesis that HZ vaccination provides broader health benefits and may help reduce the burden of serious hepatobiliary diseases in the aging population.
Comparisons with previous studies
Several studies have suggested a potential association between VZV infection and hepatobiliary outcomes, including acute hepatitis, fulminant hepatic failure [10,11], acute pancreatitis [12], and liver cirrhosis [13]. However, these studies primarily focused on infection-related complications rather than on the potential protective effects of HZ vaccination. Moreover, the existing evidence is largely derived from case reports and case-control studies with short-term follow-up, offering lower levels of evidence. In contrast, our study provides a novel contribution by investigating the association between HZ vaccination and hepatobiliary health over a longer term.
Some long-term studies have investigated the risk of VZV infection itself or primary complications of infection, such as HZ ophthalmicus and postherpetic neuralgia [6,19], highlighting greater vaccine efficacy in younger individuals and females, with protection lasting five to eight years. Nonetheless, their primary outcomes are localized complications rather than broader systemic benefits. While these studies primarily addressed localized outcomes, our findings expand the scope by emphasizing the vaccine’s potential systemic benefits, including hepatobiliary outcomes. By shifting the focus from immediate and localized complications to long-term systemic outcomes, this study adds new insights into how HZ vaccination may contribute to hepatobiliary health. Our findings underscore the importance of considering vaccination for its well-established role in preventing acute complications and its potential to mitigate chronic disease progression and broader inflammatory effects.
Plausible underlying mechanisms
The observed association between HZ vaccination and reduced hepatobiliary disease risk may be attributed to its modulation of systemic inflammation and immune dysregulation caused by VZV reactivation [20]. HZ vaccination enhances T-cell-mediated immunity and induces transient increases in IL-6, IFN-γ, and IL-2 without inducing chronic inflammation, thereby supporting immune homeostasis and reducing inflammatory burden on the hepatobiliary system [21,22]. In contrast, HZ infection triggers systemic inflammation [20], vascular injury [5], and hepatic microcirculatory damage, all of which may contribute to hepatic fibrosis, hepatic decompensation, and biliary complications. In addition, acute HZ-related stress responses, including increased catecholamine release and autonomic dysfunction [23], may impair gallbladder motility and bile acid metabolism, contributing to cholecystitis and cholangitis.
In this study, the protective benefits of HZ vaccination peaked at one to four years after vaccination and diminished after eight years, likely reflecting waning immunity and the natural progression of hepatobiliary diseases. The early peak may result from preventing acute VZV-triggered exacerbations in individuals with or without subclinical liver disease [24,25]. For conditions such as hepatic failure and cirrhosis, which evolve gradually or worsen with infection [26,27], early immune protection may delay progression. For cholelithiasis, risk reduction was most prominent in the first two years post-vaccination, and a U-shaped pattern was noted for cholecystitis and cholangitis. This may also reflect the natural progression of gallstone disease, where asymptomatic stones eventually become large enough to cause complications. As expected with live vaccines, the protective effect gradually declined, leading to an increased disease risk beyond this period.
Clinical and policy implications
HZ vaccination could prevent shingles and even reduce the burden of serious hepatobiliary diseases in the older population, broadening its value. Clinicians and policymakers typically assess zoster vaccination based on its role in preventing shingles; however, our findings suggest additional benefits, including reducing the incidence of hepatobiliary diseases. Given the substantial healthcare costs of these conditions, even a modest reduction could have significant public health implications. Patients with risk factors for liver disease (e.g., chronic viral hepatitis, alcohol use, or metabolic syndrome) might particularly benefit from vaccination. This study also suggests that individuals with unhealthy lifestyles or low socioeconomic status may derive even greater advantages. Ensuring high vaccine uptake in these groups could help prevent acute zoster and even severe liver disease progression. From a public health perspective, our findings support the expansion of HZ immunization programs. As aging populations face rising rates of nonalcoholic fatty liver disease and gallstone disease, the indirect benefits of vaccination could be meaningful. Additionally, with the advent of the recombinant HZ vaccine offering greater than 90% efficacy and longer-lasting immunity [28,29], revaccination policies may be considered to sustain these benefits. Future studies should further investigate these associations through cohort analyses and mechanistic research.
Limitations
This study has several limitations. First, as an emulated target trial, residual confounding from unmeasured factors, such as dietary habits, which influence both gallstone risk and metabolic dysfunction-associated steatotic liver disease, may exist. Second, despite excluding individuals with prior hepatobiliary events, limited data availability before 2012 and possible underdiagnosis could have introduced bias. Third, “healthy vaccine bias” may have affected results, as vaccinated individuals may engage more in health-promoting behaviors or utilize healthcare services more frequently, potentially inflating the observed protective effects [30]. Fourth, the study population was restricted to South Korea, which may limit the generalizability to populations with different healthcare systems, vaccination policies, or characteristics. To account for the heterogeneity within the study population, we assessed the representativeness of the study participants [31], as shown in Supplementary Table 2. Fifth, the number of individuals followed beyond 8 years was relatively small, reducing statistical power to evaluate long-term effects. Sixth, this study focused on the live-attenuated HZ vaccine, while emerging evidence suggests that the recombinant vaccine may also offer systemic benefits beyond preventing VZV infection [32]. However, since the recombinant vaccine was introduced in South Korea in 2022 [7], we limited our analysis to the live-attenuated vaccine to ensure an adequate follow-up period for assessing long-term outcomes. Thus, further investigation with extended follow-up is needed to elucidate their long-term effectiveness.
Conclusions
HZ vaccination is associated with a reduced risk of developing hepatobiliary diseases, mainly hepatic failure and cirrhosis, suggesting benefits beyond shingles prevention. This protective association lasted for up to eight years, with the most pronounced risk reduction within the first four years. These findings suggest that reducing VZV infection may indirectly lower the risk of severe morbidity associated with hepatobiliary diseases through vaccination. If validated in further studies, this could help shape vaccination strategies to prevent hepatobiliary complications.
KEY MESSAGE
1. Live zoster vaccination was associated with a reduced risk of hepatobiliary and pancreatic diseases.
2. The protective effect persisted for up to 8 years, peaking at 1–4 years after vaccination, consistent with the duration of live herpes zoster vaccine immunity.
3. Benefits were consistent across subgroups, with greater effects in males, younger adults, and those with unhealthy lifestyles.
Notes
Acknowledgments
We thank the Korea Healthcare Bigdata of the Ministry of Health and Welfare (South Korea) for providing the data in 2022 (2022-00061).
CRedit authorship contributions
Junyeol Kim: writing - original draft; Kyeongmin Lee: methodology, investigation, formal analysis, writing - original draft, visualization; Jiyeon Oh: methodology, writing - review & editing; Hayeon Lee: conceptualization, methodology, visualization; Jong-In Chang: writing - review & editing; Tae Young Park: writing - review & editing; Dong Keon Yon: conceptualization, methodology, formal analysis, writing - review & editing, supervision, funding acquisition; Hyoung-Chul Oh: writing - review & editing, supervision
Conflicts of interest
The authors disclose no conflicts.
Funding
This research was supported by the MSIT (Ministry of Science and ICT), Korea, under the ITRC (Information Technology Research Center) support program (IITP-2024-RS-2024-00438239) supervised by the IITP (Institute for Information & Communications Technology Planning & Evaluation).The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Data availability
The data are available upon request. Study protocol and statistical code: Available from DKY (yonkkang@gmail.com). The dataset was available from the Korean National Health Insurance Service (NHIS), Korea Health Insurance Review and Assessment Service (HIRA), and the Korea Disease Control and Prevention Agency (KDCA) through a data use agreement.