Resistance to Thyroid Hormone with Missense Mutation (V349M) in the Thyroid Hormone Receptor Beta Gene
Article information
Abstract
Resistance to thyroid hormone (RTH) is a syndrome characterized by reduced sensitivity to the thyroid hormone. It is generally caused by mutations in the thyroid hormone receptor β (TRβ) gene. On the basis of its clinical features, two different forms of this syndrome have been described: generalized resistance and pituitary resistance. A total of 122 TRβ gene mutations have been identified thus far. A 38-year-old woman presented with intermittent palpitation. Thyroid function tests revealed elevated levels of free T4 and TSH. TSH α-subunit levels were 0.41 mIU/mL, and magnetic resonance images of the sellar region evidenced no abnormal findings. The TSH response to TRH stimulation was found to be normal. The sequence analysis of the TRβ gene verified a missense mutation in exon 11, and the observed amino acid alteration was a substitution of a valine for a methionine at codon 349. We report the first case of a woman with RTH, which was found to be caused by a missense mutation (V349M) in the TRβ gene.
INTRODUCTION
Thyroid hormone resistance (RTH) syndrome is a rare disorder, which is characterized by reduced target tissue responsiveness to circulating free-thyroid hormones. Thyroid hormone resistance was initially described by Refetoff et al. in 19671). Typically, patients evidence elevated serum levels of free T4 and T3 associated with normal or slightly elevated TSH2). On the basis of the clinical features, at least two different forms of RTH have been described: generalized resistance to thyroid hormone (GRTH), in which patients are asymptomatic with a few clinical signs, and pituitary resistance to thyroid hormone (PRTH), in which patients present with some thyrotoxicosisassociated signs and symptoms. However, there is a wide overlap in the symptoms and signs evidenced by individuals with GRTH and PRTH3). Although the clinical expression of this syndrome is heterogenous, goiter is the most common clinical finding which brings the individual to medical attention3), and the classic features include attention-deficit hyperactivity disorder, delays in growth, and tachycardia1). Resistance to thyroid hormone is generally transmitted in an autosomal dominant manner. However, sporadic de novo cases are also common, although recessive inheritance is rare1). The linkage between RTH and the TRβ gene was elucidated in 19884). Since that time, approximately 100 mutations have been detected in this gene1, 5), which are clustered primarily in hot spots in the T3-binding domain (exons 8, 9 and 10)6-8). In this study, we report the first case in Korea of a woman with PRTH caused by a missense mutation (V349M) in the TRβ gene.
CASE REPORT
A 38-year-old Korean woman was referred to us in June 1998, complaining of intermittent palpitation that had persisted for 2 years. Her treatment was initiated under the diagnosis of hyperthyroidism in June 1996 at a primary care clinic, and after a six-month treatment, she discontinued medication. When first seen at our clinic, she weighed 53 kg, her height was 158 cm, her blood pressure was 130/80 mmHg, and her pulse rate was 90 beats/min. The patient had no family history of thyroid diseases. The patient's thyroid gland was diffusely symmetrically enlarged, and no thrill, bruit, or exophthalmos was detected. At that time, her thyroid function tests revealed free T4, 2.60 ng/dL (range, 0.7-1.8); T3, 150 ng/dL (range, 80-200); TSH, 3.0 µIU/mL (range, 0.1-4.2); anti-thyroglobulin (TG) antibody, 20.34 U/mL (<0.3); and anti-microsomal antibody, 11.30 U/mL (<0.3).
After three visits, the patient was lost to follow-up. In December 2004, she visited a primary care clinic, and her thyroid function tests revealed free T4, 2.51 ng/dL; T3, 163 ng/dL; and TSH 14.24 µIU/mL. In February 2005 she received an anti-thyroid drug, methimazole 10 mg, due to suspicion of diffuse goiter. She was again referred to us in July 2005 for further evaluation and treatment for diffuse goiter. Thyroid function tests, which were conducted at a primary care clinic in March 2005, revealed free T4, 1.89 ng/dL; T3, 224 ng/dL; and TSH, 33.59 µIU/mL. We then discontinued methimazole, and the patient was scheduled to take thyroid function tests after one month. Thyroid function tests were conducted one month later, and revealed the following: free T4, 3.53 ng/dL; T3, 300 ng/dL; TSH, 3.0 µIU/mL; and thyroxine-binding globulin (TBG), 23.08 µg/mL (range, 11.3-28.9). Tests for thyroid autoantibodies revealed TG antibody, 68.04 U/mL; anti-microsomal antibody, >100 U/mL; thyroid stimulating immunoglobin (TSI), 0% (<15); and T3 and T4 autoantibodies, negative. Thereafter, the patient was treated with atenolol 100 mg for 4 weeks. Four weeks later, she had no palpitation and her medication was discontinued. Thyroid scans indicated that the thyroid gland was diffusely enlarged, but we noted no abnormal focal lesions (Figure 1). Basal serum TSH levels were abnormally high, and increased to a significant degree after stimulation with 200 µg of TRH (Table 1). The level of TSH -subunit was 0.41 mIU/mL (range, 0-0.9), TSH α-subunit/TSH was 1, and sex hormone-binding globulin (SHBG) was 39.23 nmol/L (range, 30-100). MR imaging of the sellar lesion evidenced no abnormal findings (Figure 2). For the next diagnostic plan, we conducted a sequence analysis of the TRβ gene. The result revealed a missense mutation in exon 11 and an amino acid alterationnamely, a substitution of valine for methonine at codon 349 (Figure 3). On the basis of these results, she was ultimately diagnosed with thyroid hormone resistance syndrome, and she has been followed up with periodic thyroid function tests.
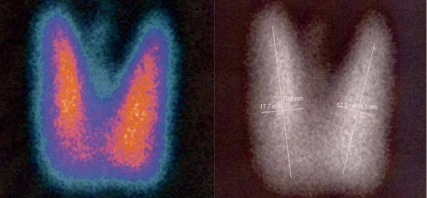
99mTechnetium thyroid scan reveals diffuse enlargement of both thyroid glands without abnormal focal lesions.
DISCUSSION
Thyroid hormone resistance syndrome is characterized by elevated levels of circulating free thyroid hormone, inappropriate TSH secretion, and reduced peripheral tissue responses to the activity of iodothyronine. Inappropriate secretion of TSH and the coexistence of elevated serum thyroid hormone and normal TSH levels has been noted in several situations, including abnormal thyroid hormone transport, TSH-secreting pituitary neoplasms, and pituitary or peripheral resistance to thyroid hormones9, 10.
RTH is the consequence of mutations in the gene of the thyroid hormone receptor (TRs). The mutant TR interferes with the activity of normal TR to induce the clinical syndrome11). As RTH patients present with elevated thyroid hormone levels and goiter, frequently accompanied by some manifestations of thyrotoxicosis, the condition is often misdiagnosed as Graves' disease. However, exophthalmos has never been identified as a feature of RTH, and thyroid auto-antibodies have been detected only in approximately 4% of patients12). By way of contrast, thyroid hormone hypersecretion as the result of stimulating auto-antibodies (Graves' disease) or hyperfunctioning thyroid tissue (autonomous single or multinodular goiter) is uniformly associated with the inhibition of TSH secretion, resulting in unmeasurable serum TSH levels13).
Clinically, thyroid hormone resistance can be divided into two entities: GRTH and PRTH11). Both GRTH and PRTH are rare diseases, occurring at any age and with no sex predilection. In general, diagnoses of PRTH are made largely on the basis of the presence of the clinical signs and symptoms of hyperthyroidism13). Comprehensive assessments utilizing biochemical and physiological indices of thyroid hormone action are useful, but are limited by their lack of precision. Moreover, they also evidence an overlap between the values recorded in cases of GRTH and PRTH11). No differences in the absolute levels of TSH or free thyroid hormone are observed in GRTH patients as opposed to PRTH patients. Stimulatory and inhibitory tests have also been conducted in GRTH and PRTH patients, in order to evaluate the pituitary-thyroid axis in a dynamic manner. The TSH response to TRH is normal, or even exaggerated, and is prolonged in all GRTH and PRTH patients2). A molecular mechanism to explain these two clinical phenotypes has proven elusive, and many authors have concluded that they are part of a spectrum of the same disorder13).
TRs belong to the superfamily of ligand-dependent transcription factors. TRs are encoded by the thyroid hormone receptor α gene (THRA) and the thyroid hormone receptor β gene (THRB), which are located on chromosomes 17 and 3, respectively. The expression of TRs occurs in a tissue-dependent fashion. Although THRA1 and THRB1 are expressed ubiquitously, THRA1 is expressed predominantly in the heart, bone, and brain, whereas TRβ1 is expressed more abundantly in the liver, kidney, and thyroid. THRB2 expression is limited to the pituitary gland, hypothalamus, retina, and inner ear, and THRB3 expression occurs principally in the heart and kidney14). One of the most salient reasons for recognizing RTH is that its management differs from that of other common forms of thyroid dysfunction. In addition, discrimination between GRTH and PRTH using clinical criteria remains useful, as the management of the two states differs. Patients with unequivocal peripheral or pituitary resistance should not be treated with antithyroid agents, as any reduction in elevated thyroid hormones may aggravate symptoms, reduce growth and development, and exacerbate goiter. Patients who present with apparent selective pituitary resistance are the most difficult to manage. Many of the patients with peripheral resistance appear to benefit from further increases in serum thyroid hormone levels as the result of cautious exogenous administration of T4 or T3. At a minimum, such therapy tends to reduce serum TSH levels and goiter to some degree15).
With the increasing availability of sensitive and specific TSH radioimmunoassys, a greater number of these patients are being detected. It is important to remember that these patients may display only minimal elevation of serum TSH. On rare occasions, TSH may be within normal ranges, but may still be inappropriately high, despite elevated free thyroid hormone levels.