The Prevalence of Antithyroid Autoantibodies in Normal Korean Population*:
Age, Sex Distribution and Its Relation to Thyroid Function
Article information
Abstract
The prevalence of antithyroid autoantibodies and the relationship between the presence of autoantibodies and thyroid functions were studied in 848 apparently normal Korean adults with tanned red cell agglutination technique. Results are summarized as follows: 1) The prevalence of antimicrosimal antibody (MCHA) and antithyroglobulin antibody (TGHA) were 4.4% and 1.9% in 458 males, and 12.4% and 5.0% in 390 females, respectively. Both autoantibodies were more prevalent in female (p<0.001, p<0.01). 2) The age-specific prevalence of MCHA was 4.0% in their twenties, 10.1% in their thirties, 12.5% in their forties, 12.0% in their fifties, 8.3% over sixty, and those of TGHA were 2.0% in their twenties, 3.0% in their thirties, 7.0% in their forties, 4.2% in their fifties, 2.5% in subjects over sixty, respectively. Both showed maximal values around forty and fifty and tended to be lower thereafter. 3) Mean T3, T4 and TSH values of high titer group (⩾1:1002) (n=32) were 125 ± 20.6 ng/dl, 9.1 ± 1.7μg/dl and 4.0 ± 1.8 uU/ml, and those of low titer group (<1:1002) (n=44) were 134 ± 24.3 ng/dl, 9.6 ± 1.7 ug/dl and 3.2 ± 1.2 νU/ml. T3 was lower and TSH, higher in high titer group than in low titer group (p<0.05, p<0.05), and no significant difference was observed in T4 level (p<0.1). In conclusion, the prevalence of MCHA and TGHA were higher in apparently normal females than in males with their peaks around forty and fifty, being lower thereafter, and antithyroid autoantibody of high titer (⩾1:1002) was related to alteration of thyroid functions suggesting the existence of “subclinical autoimmune thyroiditis” state.
INTRODUCTION
Although antithyroid autoantibody has been recognized as a diagnostic indicator and pathogenetic factor as it is elucidated that autoimmunity plays a crucial role in the development of various thyroid diseases, there are many controversies regarding the mechanism of genesis of autoantibodies and their role in the body.1–8)
Meanwhile, it was found the antithyroid autoantibodies are present in the apparently normal person to make the clinical significances of antithyroid autoantibodies more ambiguous, and it is not known whether their presence in the normal subjects means subclinical autoimmune thyroid disease which move to clinically overt thyroid diseases. 10–13)
The present investigation was done to study the prevalence of antithyroid autoantibodies in normal persons according to age and sex distribution with its relation to thyroid functions.
MATERIALS AND METHODS
The normal sera were obtained from 848 apparently normal Koreans who visited Seoul National University Hospital, Choonchun Prefectural Hospital and Masan Prefectural Hospital from March 1984 to June 1984 for pre-employment of in-job routine check-up and were regarded to the free from previous or present thyroid disease after physical examination and checking past medical history. They comprised 458 men and 390 women, and according to age distribution, 199 were in their twenties (M:100, F:99), 198 were in their thirties (M:99, F:99), 200 were in their forties (M:100, F:100), 143 were in their fifties (M:100, F:43), and 108 person were over sixty (M:59, F:49).
The presence of anti-thyroglobulin antibody (TGHA) and anti-microsomal antibody (MCHA) were checked with Thyroid Test® and Microsome Test® (Fuji Zoki Co.) using tanned red cell agglutination method developed by Fulthorpe et al.14) and Roitt et al.15) respectively. The test was considered to be positive when red cell agglutination over 1 + occurred at the dilution titer above 1:102, 16, 17)
Thyroid function tests were done for the 76 subjects who showed positive results with MCHA and/or TGHA test, and 75 subjects who were selected randomly from the persons who showed negative results with both tests (5 males and 10 females at each age group). T3 resin uptake was determined with Triobead® (Abbott Lab.), and serum thyroxine (T4), triiodothyronine (T3) and TSH levels were measured with radioimmunoassay using Tetrabead™-125® (Abbott Lab.), T3 RIA Bead™® (Abbott Lab.) and Daiichi kit, respectively.
The statistical evaluations were made with Student’s t-test, χ2-test and ANOVA with one-way classification, and p-value below 0.05 was regarded as significant. The results of prevalence study were corrected on the assumption that composition of population with relation to age and sex distribution in the present study is identical to that presented in Korea statistical year book, 1980 to obviate the bias due to arbitrary allocation of subject number to the respective age and sex group18, 19)
RESULTS
The prevalence of antithyroid autoantibodies in normal subjects
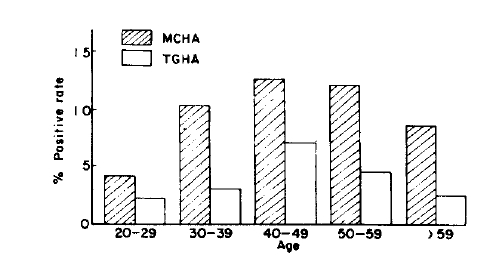
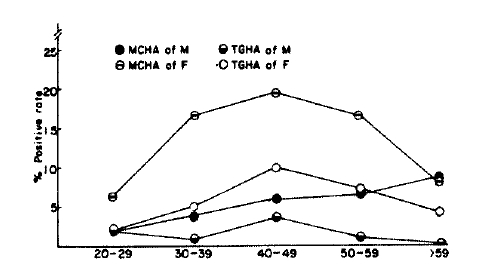
The prevalence of MCHA was 4.4% in male adults and 12.4% in female adults, and those of TGHA were 1.9% in male and 5.0% in female, respectively. Both autoantibodies were more prevalent in female adults (p<0.001, p<0.01) (Table 1). With regard to age-specific prevalence of antithyroid autoantibodies, those of MCHA were 4.0% in their twenties, 10.1 % in their thirties, 12.5% in their forties, 12.0% in their fifties, 8.3% their over sixty, and those of TGHA were 2.0% in their twenties, 3.0% in their thirties, 7.0% in their forties, 4.2% in their fifties, 2.5% in the age group over sixty, respectively. Both showed increasing values in accordance with advancing age with their peaks in the forties, and tended to be lower thereafter (Fig. 1, Table 1). In the aspect of both age and sex specific prevalence of antithyroid autoantibodies, all showed their peak values in the forties, except MCHA in the male which showed a progressive increment even after forty (Table 1, Fig. 2).
Relationship between the presence of antithyroid autoantibody and thyroid functions
In 76 subjects with positive MCHA and/or TGHA tests, serum T3 levels was 131 ± 23.2 ng/dl (mean ± S.D.), T4, 9.4 ± 1.7 νg/dl, TSH, 3.5 ± 1.5 νU/ml, and in 75 subjects with both negative MCHA and TGHA tests, T3 was 135 ± 21.2 ng/dl, T4, 8.4 ± 1.7 ug/dl, TSH, 3.2 ± 1.2 νU/ml. Serum T3 and TSH levels did not show any significant difference between the two groups (p>0.1, p>0.05), but T4 level was significantly higher in the autoantibody-positive group (p<0.01) (Table 2, Fig. 3). Meanwhile, thyroid functions did not show any significant change with relation to age in each autoantibody-positive and negative group (p>0.05 in all cases) except the T4 level in autoantibody negative male (F-ratio; 4.43, p<0.05) (Table 3, Table 4). In the autoantibody-positive subjects, 2 (2.6%), 9 (11.8%) and 8 cases (10.5%) showed values above mean + 2 S.D. of T3 T4 and TSH values of autoantibody-negative subjects, respectively, and there was no case below mean-2 S.D. Especially, 6 out of 8 cases whose TSH levels were above mean + 2 S.D. of level of autoantibody-negative subjects showed high titer (⩾1:1002) of autoantibodies, and 18.8% (6/32) of subjects with high titer (⩾1:1002) of autoantibodies and 4.5% (2/44) of subjects with lower titer (<1002) of autoantibodies showed serum TSH levels above mean + 2 S.D. of autoantibody-negative subjects.
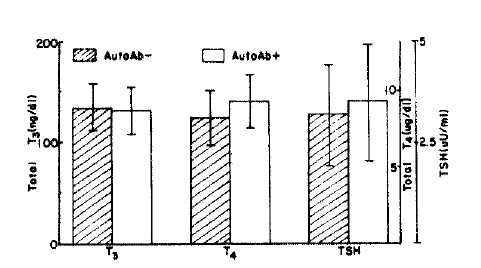
Thyroid function of autoAb positive and negative groups: mean ± S.D. significant difference present in T4.
Thyroid functions in relation to titer of autoantibody
In 32 subjects whose autoantibody titer was equal to or over 1:1002, serum T3 level was 125 ± 20.6 ng/dl, T4, 9.1 ± 1.7 νd/dl, TSH, 4.0 ± 1.8 νU/ml, and in 44 subjects whose titer was below 1:1002 (but equal to or over 1:202), serum T3 level was 134 ± 24.3 ng/dl, T4, 9.6 ± 1.7 νg/dl, TSH, 3.2 ± 1.2 νU/ml. T3 level was higher in the latter group and TSH was higher in the former group, while, T4 did not show any significant difference between the two groups.
DISCUSSION
The prevalence of antithyroid autoantibodies in the normal population has been reported to be from below 1%12) to over 50%20) according to investigators although it is generally accepted that the prevalence is higher in females, and that of anti-thyroglobulin antibody is lower than that of anti-microsomal antibody. This variation in reported prevalence seems to be attributable to the differences in the method of detection of autoantibodies, materials, and criteria of positive tests etc. Our results (MCHA:4.4% in males, 12.4% in females, TGHA: 1.9% in males, 5.0% in females) bear resemblance to the report of Notsu et al.21) (MCHA: 5.6% in males, 11.1 % in females, TGHA: 1.4% in males, 3.8% in females) who used similar methods to ours in detection of autoantibody and in criteria of positive tests. There seems to be no bias due to arbitrary allocation of sample numbers in our investigation because we corrected our data as described above. Bjro et al.10) and Turnbridge et al.12) reported increasing prevalence of antithyroid autoantibodies with advancing age, whereas, Hawkins et al.13) reported declining prevalence of antimicrosomal antibodies in females after fifty, which is similar to our results showing a decrease in prevalence of both MCHA and TGHA after fifty. The clinical and immunological meanings of age-related changes in prevalence of antithyroid autoantibodies are obscure at present because mechanism of genesis of antithyroid autoantibodies itself is far from clear.
A new emergence of antithyroid autoantibody-producing clone or an anamnestic reaction of preexistent undetected clone to thryoid antigens with increased leakage into circulation with aging might partly explain the increased prevalence in the aged group. Disturbance of intricate immune modulation with aging can also be a possible mechanism to explain the above findings, whereas, the declining propensity of prevalence after fifty observed in several studies including ours can be ascribed to the diminished function of immune system itself, or disturbance of hemagglutination test by the presence of blocking-type antibody22) or thyroglobulin in bloodstream23) in the elderly. Because we performed hemagglutination tests with all dilution titers from 1:102 to 1:12802 in every case, the possibility of disturbance due to the presence of blocking-type antibody is nearly absent, but we could not exclude in the present study the possibility that released thyroglobulin in the elderly might hinder hemagglutination reaction to lower the titer of autoantibodies in over fifty.
It must also be kept in mind that peak prevalence of antithyroid autoantibodies in the forties cannot be directly related to the peak incidence of autoimmune thyroid diseases around forty24, 25) because the indices we investigated are not incidence but prevalence of antithyroid autoantibodies.
The exact clinical meaning of the presence of antithyroid autoantibodies in normal subjects is still unknown. Bjøro et al.10) and Gordin et al.26) insist the state of “symptomless autoimmune thyroiditis” in which antithyroid autoantibodies are present with elevated TSH level but without clinical symptom, and Gordin et al.27) and Khangure et al.28) reported that 6–28% of apparently normal persons with “symptomless autoimmune thyroiditis” developed clinically overt hypothyroidism during follow-up for several years. With our results the TSH level was significantly higher, and the T3 level, significantly lower in the high titer group than in the lower titer group, it could be argued that the high titer group may be in the state of clinically inapparent hypothyroidism, and 6 subjects whose TSH levels were over mean + 2 S.D. and autoantibody titers were equal to or over 1:1002 may be categorized to “symptomless autoimmune thyroiditis” as insisted by Gordin et al.26). These findings suggest that antithyroid autoantibodies of high titer may be the reflection or cause of damage to thyroid gland by autoimmunity, and are consistent with the finding that antimicrosomal antibody is well correlated with histopathologic findings in Hashimoto’s disease.29) We arbitrarily regarded titers equal to or over 1:1002 as high titer, considering the distribution of titers in those who showed TSH levels over mean + 2 S.D. of autoantibody-negative group. But a higher T4 level in autoantibody-positive group could not be explained on the above assumptions, and it may be accounted for by the possibility that the subjects associated with previous or present inapparent Graves’ disease might be included in our study.
Thyroid functions were found to bear little relationship with age in respective autoantibody-positive and negative groups, and this finding suggests aging per se is not a direct cause of diminished thyroid functions. Age-related increase of serum TSH level in the general population noticed by other investigators12) is, instead, due to high proportion of autoantibody-positive subjects with advance in age, and it was also found by others12) that if autoantibody-positive subjects were excluded, no age dependent change in TSH level was found.
The proportion of the cases with antithyroid autoantibodies of high titer (⩾1:1002) in the autoantibody-positive group were 40.8% (31/76) and it was not significantly lower than 40% (72/181) in Graves’ disease and 57% (60/106) in Hashimoto’s disease30) to be contradictory with contention that antithyroid autoantibody was present in low titer if present in normal persons.31)