Microsatellite Instability in Korean Patients with Gastric Adenocarcinoma
Article information
Abstract
Objectives
Microsatellites are short repeated oligonucleotide sequences found throughout the human genome. High mutation rates in microsatellite sequences have been found in tumors from patients with hereditary non-polyposis colorectal carcinoma and some sporadic carcinomas. However, little information is available regarding RER-positive phenotype in gastric carcinomas, particularly in terms of age of onset and other pathologic features, such as histologic types, degree of differentiation, location or stage of the carcinoma.
Methods
To obtain a better understanding of the molecular mechanism of gastric carcinogenesis, microsatellite instability was examined at 6 gene loci (D2S71, D2S119, D3S1067, D6S87, D8S87, D11S905) in 77 gastric carcinomas (40 cases of young patients and 37 cases of elderly patients).
Results
RER-positive phenotypes were found in 17 (22.1%) of 77 cases. In young patients (under 40 years) RER-positive phenotype was found in 9 (22.5%) of 40 cases, and in elderly patients 8 (21.6%) of 37 cases. Moderately differentiated carcinoma revealed a significantly high frequency of RER-positive phenotype than well differentiated carcinoma (p<0.001). Tumors arising from the middle third (p<0.001) or lower third (p<0.001) revealed higher frequency of RER-positive phenotype than the tumors arising from the upper third of the stomach. The RER-positive phenotype was not significantly affected by the sex, histologic type or stage of carcinoma.
Conclusion
RER-positive phenotype occurs frequently in gastric carcinoma, although the frequency of RER-positive phenotype between young and elderly patient was not significantly different. Thus, the acquisition of RER-positive phenotype might be an early event in gastric carcinogenesis.
INTRODUCTION
Microsatellite is a tandem array of short stretches of nucleotide sequences, usually repeated between 15 and 30 times1,2). These regions of genome tend to be polymorphic or variable among individuals. Recently, abnormalities of such repeats have been implicated in the genesis of hereditary non-polyposis colorectal cancer (HNPCC). In HNPCC kindreds, linkage to the marker D2S123 on chromosome 2p was reported, and it was simultaneously noted that instability of the microsatellite repeat number was present in microsatellites scattered throughout the genome3,4). It was suggested that the mutation affecting DNA replication or repair predisposed to replication errors. Candidate gene of mismatch repair in gene hMSH2 was identified and shown to be a DNA mismatch binding protein5,6). Subsequently, additional mismatch repair genes were shown to be involved in the pathogenesis of HNPCC, comprising hMLH1, hPMS1, and hPM27,8). Analysis of sporadic tumors belonging to the HNPCC spectrum (colorectal carcinoma, endometrial carcinoma and gastric carcinoma) revealed a significant proportion of cases with multiple replication errors, as in HNPCC cases9). In contrast, other sporadic tumors (lung, breast, testis, CNS tumors and soft tissue sarcomas) showed microsatellite instability (MI) as a rare event10). Recent reports show that cases of small cell carcinoma of the lung, cases with multiple primary tumors and late stage chronic myelocytic leukemia may be associated with replication error (RER)-positive phenotype11–13). Presumably, it has undergone a germ line mutation of mismatch repair genes in HNPCC; similarly somatic mutations of mismatch repair genes are presumed to have occurred in the tumors of some sporadic colon cancer14).
Gastric adenocarcinoma is one of the most common malignancies in the world, especially in Korea. Although the molecular basis of the development of gastric carcinoma remains unclear, there have been many attempts to apply the same analysis which has been effective in identifying the oncogenesis of colon cancer15,16). However, little information is available regarding RER-positive phenotype in gastric carcinomas, particularly in terms of age of onset (gastric carcinoma of the young vs. elderly patient) and other pathologic features such as histologic types, degree of differentiation, location, and stage of the carcinoma. Gastric carcinoma of the young adult is characterized by mostly diffuse (by Lauren’s criteria17) or signet-ring cell type (by WHO criteria18), poorly differentiated adenocarcinoma without adjacent intestinal metaplasia, and high incidence of family history of gastric carcinoma19–21). In order to obtain a better understanding of the molecular mechanism of gastric carcinogenesis, microsatellite instability was examined at 6 gene loci in 77 gastric carcinomas (40 cases of young patients and 37 cases of elderly patients).
MATERIALS AND METHODS
1. Materials
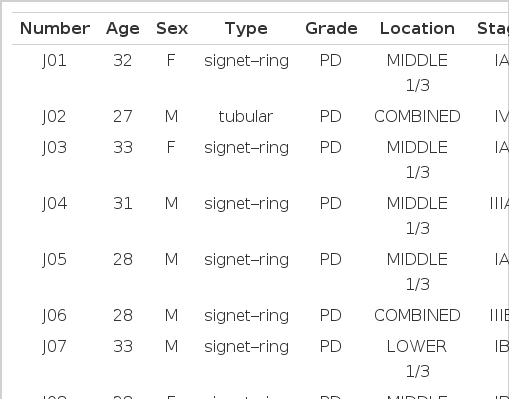
Forty cases of subtotal or total gastrectomy specimens received at the Department of Pathology of Chonnam University Hospital (69 cases) and Presbyterian Medical Center (8 cases), which were diagnosed as primary adenocarcinoma of stomach in the interval of January 1, 1990 to May 1, 1996, were selected. The adenocarcinomas were classified according to the WHO criteria18), and clinicopathological data were collected from the review of medical records (Table 1). Males were 42 cases, and females were 35 cases. Tubular adenocarcinoma was 36, signet-ring cell type was 36 and mucinous type was 5 cases; well differentiated adenocarcinoma was 6, moderately differentiated was 21 and poorly differentiated was 50 cases. Four cases were located in the upper third of the stomach (cardia and fundus), 25 cases in the middle third (body) and 30 cases in the lower third (antrum and pylorus). Twelve cases were located in two different areas, and 6 cases were undetermined. Stage Ia was 13 cases, Ib 9, II 13, IIIa 13, IIIb 5, and IV 18. Stages were undetermined in 6 cases.
2. DNA isolation from the tissue
A hematoxylin-eosin stained tissue section from each block was used to determine the area of highest tumor cellularity for DNA preparation. One or more tissue plugs were then removed from each block, minced with a razor blade and deparaffinized with a series of rinses in xylene and ethanol. The tissue was digested completely in TE buffer (pH 8.0), containing 400 μg/ml of proteinase K replenished daily and 0.5% sodium dodecyl sulfate, for 3–4 days at 54°C. Following a standard series of phenol, phenol/chloroform and chloroform extractions, the DNA was ethanol-precipitated, washed in 70% ethanol, resuspended in TE buffer (pH 8.0) and stored at −20°C.
3. PCR analysis at microsatellite loci
The primers used to examine the microsatellite loci were: D2S71, D2S119 (AFM077yb7)22), D3S106723), D6S87 (MFD47)24), D8S87 (MFD39)25), and D11S905 (AFM105xb10)22). The PCR was performed in 10μl volumes of a mixture containing 1x PCR buffer [10mmol Tris-HCI, pH 8.8, 1.5mmol MgCl2, 50 mmol KCI, 0.1% Triton X-100], 10pmol of unlabeled primer and 5pmol of labeled primer with [γ-32p]ATP(>5,000ci/mmol), 50ng DNA, 0.1 units Taq DNA polymerase, 125μmol of each deoxynucleotide. Reaction mixtures were heated to 95°C for 5 min and cycled 32 times; each cycle consisted of 1 min at 94°C, 1 min at 52–58°C, 1 min at 72°C for strand elongation. After that, heated for 10 min at 72°C for the final elongation using a PCR machine (Model 480, Perkin Elmer Cetus, Norwalk, CT, USA). Following PCR, 3μl of reaction product was denatured and then electrophoresed in 6% polyacrylamide gels containing 8 mol urea. After electrophoresis, the gels were exposed to X-ray film for 3–6 h at −70°C.
4. Interpretation of PCR results
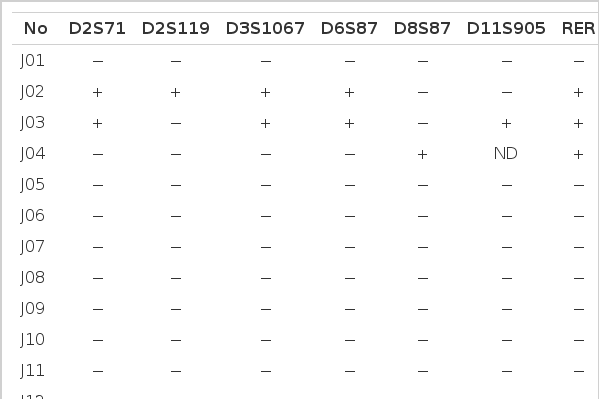
Microsatellite instability (MI) was evident when the tumor DNAs gained new bands compared to their normal counterparts (Fig. 1A, B). If at least one locus revealed MI, the case was regarded as RER-positive (Table 2). Deletion of band (loss of heterozygosity of microsatellite allele) was not considered to be a microsatellite alteration in the present study.
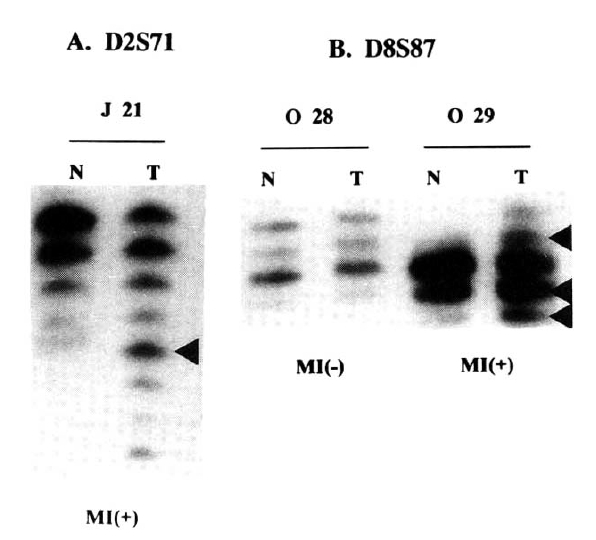
Analysis of genetic instability in paired normal (N) and tumor (T) DNA at loci D2S71 of J21 patient (A), and D8S87 of O29 and O28 patients (B). At tumor DNAs, abnormal patterns indicating expansion are shown at each microsatelite locus. Normal alleles appear as major bands with their ladders. Patient numbers are shown above the lanes.
MI(+); microsatellite-positive, MI(−); microsatellite-negative
RESULTS
Table 2 summarizes results of PCR analyses in 40 cases of the gastric carcinoma in young adult and 37 cases in elderly. Fig. 1A shows typical MI of young adult (J21) and Fig. 1B reveals heterozygosity (O28) and MI (O29) from elderly patients.
Out of 77 cases of gastric carcinoma, 17 cases (22.1%) were RER-positive and 60 cases (77.9%) were RER-negative. Nine cases (22.5%) out of 40 young adult cases were RER-positive and 8 (21.6%) out of 37 elderly patients were RER-positive (Table 3A). Seven (16.7%) out of 42 male cases revealed RER-positive phenotype, and 10 (28.6%) out of 35 female cases showed RER-positive phenotype. There was no difference in RER-positivity between the male and female patients by logistic regression analysis (Table 3B). Ten (27.8%) out of 36 tubular type, 6 (16.7%) out of 36 signet-ring cell carcinoma and 1 (20.0%) out of 5 mucinous carcinoma were RER-positive phenotype (Table 3C).
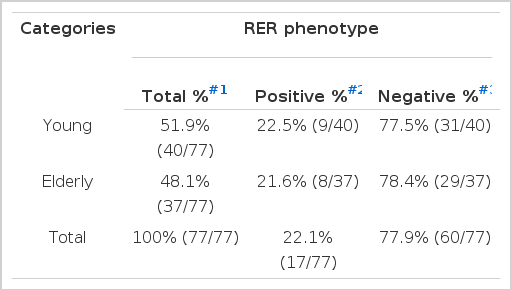
Frequency of RER-positive and Negative Phenotypes in Young and Elderly Gastric Adenocarcinoma Patients
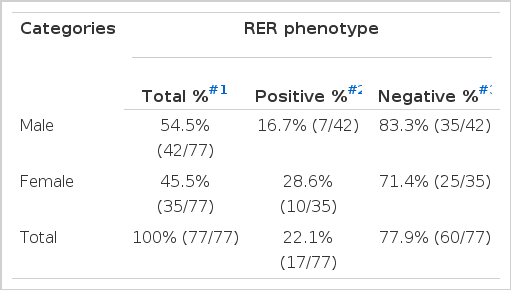
Frequency of RER-positive and Negative Phenotypes in Male and Female Gastric Adenocarcinoma Patients
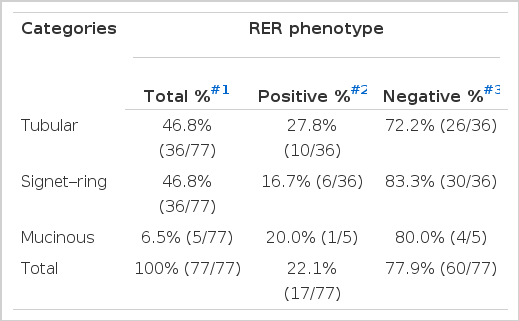
Frequency of RER-positive and Negative Phenotypes According to the Histologic Types of Gastric Adenocarcinoma Patients
One (16.7%) out of 6 well differentiated carcinoma was RER-positive, whereas 28.6% (6/21) of moderately differentiated carcinoma and 20.0% (10/50) of poorly differentiated carcinoma were RER-positive (Table 3D). Moderately differentiated carcinoma revealed higher frequency of RER-positive phenotypes than well differentiated carcinoma (p<0.01; by logistic regression analysis).
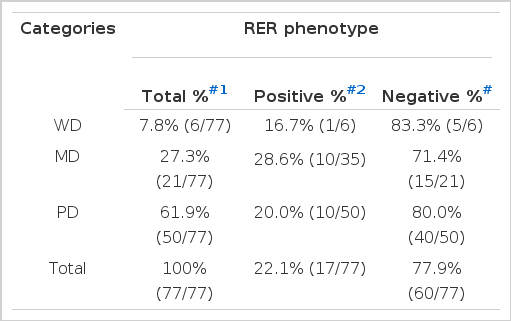
Frequency of RER-positive and Negative Phenotypes According to the Differentiation of Gastric Adenocarcinoma Patients
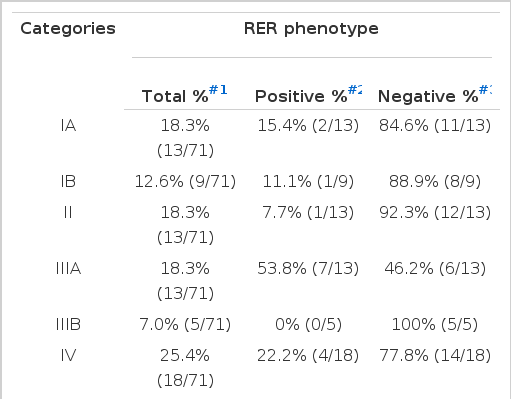
None of the tumors located in the upper third of the stomach (cardia and fundus) were RER-positive, while 16.0% (4/25) of the tumors located in the middle third (body) and 26.7% (8/30) of the tumors located in the lower third (antrum and pylorus) was RER-positive. The frequency of RER-positive cases arising from the middle (p<0.001) and lower third (p<0.0001) of the stomach was significantly higher than the tumor arising from the upper third (by logistic regression analysis) (Table 3E).
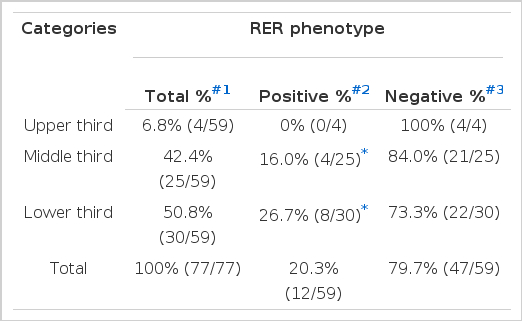
Frequency of RER-positive and Negative Phenotypes According to the Location of Gastric Adenocarcinoma
Percentage of RER-positive cases was 15.4% (2/13) in stage IA, 11.1% (1/9) in stage IB, 7.7% (1/13) in stage II, 53.8% (7/13) in stage IIIA, 0% (0/5) in stage IIIB and 22.2% (4/18) in stage IV, respectively, by logistic regression analysis (Table 3F).
DISCUSSION
It has been demonstrated that the expression of several oncogenes and the mutation of tumor suppressor genes may vary according to histologic types of gastric carcinoma and affect the biologic behavior of gastric carcinoma26,27). Recently, RER-positive phenotypes have also occurred in sporadic gastric carcinoma and played a role in tumor progression. The frequency of RER-positive phenotype ranges from 15% to 38.5%28–32). The frequency of RER-positive phenotype of this study was 22.1%, when six chromosomal loci were examined. This suggests that RER-positive phenotype is important in some portion of gastric carcinogenesis.
Gastric carcinoma of the young adult is characterized by mostly diffuse (by Lauren’s criteria17) or signet–ring cell type (by WHO criteria18), poorly differentiated adenocarcinoma without adjacent intestinal metaplasia and high incidence of family history of gastric carcinoma19–21). In the present study, the frequency of RER-positive phenotype in young patients was 22.5% compared to the 21.6% in elderly patients. When the other factors (such as sex, histologic type, degree of differentiation, location and stage) are considered, there is no significant difference of RER-positive phenotype between the two groups.
Tumors arising from the middle or lower third of the stomach revealed higher frequency of RER-positive phenotype than the tumors from the upper third (cardia and fundus). This finding supports the hypothesis that instability is tissue specific, as proximal colon carcinoma reveals higher frequency of RER-positive phenotype than distal colon carcinomas33,34). RER-positive frequency was not affected by the histologic types, although tubular type and signet-ring cell carcinomas reveal relatively high frequency of RER-positivity compared with mucinous type. Moderately differentiated carcinoma revealed higher frequency of RER-positivity compared to the well differentiated carcinoma. Han et al28) reported that poorly differentiated carcinoma reveal a higher frequency of RER-positive phenotype than well differentiated one.
The result reveals that there is no difference of RER-positive phenotypes in the tumors of different stages. Even the carcinomas confined in the lamina propria (stage Ia) reveal 15.5% (2 out of 13 cases) RER-positive phenotype. Palmirotta et al.35) found that 50% of early gastric carcinomas are RER-positive when they analyzed at 11 microsatellite markers. Semba et al.36) found RER-positive phenotypes in 33% of mucosa of intestinal metaplasia, 42% of gastric adenoma and 33% of gastric carcinoma. These findings suggest that RER-positive phenotype would occur at the early stage of gastric carcinogenesis. Conversely, Chong et al.30) reported that frequency of RER-positive phenotype is significantly higher in advanced gastric carcinoma than in early gastric carcinoma (33.3% vs. 12.0%).
In conclusion, RER-positive phenotype occurs frequently in gastric carcinoma, and is tissue specific. But, there is no difference in RER-positive phenotypes between the young and elderly gastric carcinoma patients. The acquisition of RER-positive phenotype might be an early event in gastric carcinogenesis.