Effect of Short-Term Prednisolone Therapy in Patients With Severe Chronic Type B Hepatitis
Article information
Abstract
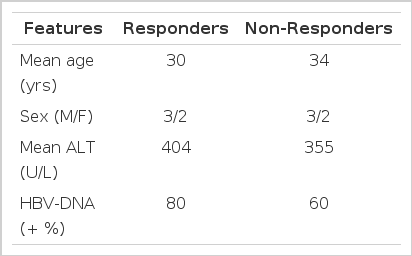
Ten patients with severe chronic type B hepatitis confirmed by liver biopsy were treated with prednisolone for eight weeks and followed up for more than one year. The patients were comprised of 6 males and 4 females, ages 17 to 45 (mean 32) yrs. Serum alanine aminotransferase (ALT) was elevated more than one month before the treatment in all (mean: 379 U/L, range: 87 to 772 U/L). Initial serological tests showed hepatitis B surface antigen (HBsAg) and hepatitis Be antigen (HBeAg) in all and hepatitis B virus DNA (HBV-DNA) in 7/10 (70%). Liver biopsy showed severe chronic active hepatitis with confluent necrosis or acinar hepatitis in all. Prednisolone, 60 mg/day, was administered initially and the dose was tapered every 2 weeks over the 8 weeks period.
Two to six months after cessation of treatment, 5 of 10 patients showed a disappearance of HBeAg and serum HBV-DNA and return of serum ALT level to normal (responders). The initial serum ALT level in responders was slightly higher than that of non-responders (mean: 404 vs. 355 U/L), but there was no statistical significance. Among 5 responders, serum HBV-DNA was detected in three patients initially and was transiently detected in one patient during treatment. In non-responders, HBeAg persisted during and after the treatment and serum HBV-DNA persisted in three, but serum ALT was decreased in all. One patient who did not show any clinical or serological improvement, died of jaundice, ascites and hepatic encephalopathy 4 months later. In all responders and in 3 of 5 non-responders who showed clinical improvement, serum ALT was repounded between the 4th and 8th week of treatment. But no rebound of serum ALT or clinical reactivation of hepatitis was observed after cessation of the drug during the follow-up period in all 10 patients. In conclusion, short-term prednisolone therapy may be effective in some patients with severe chronic type B hepatitis, but should be used selectively and cautiously. Studies with larger numbers of patients may be needed to clarify this important question.
INTRODUCTION
Chronic hepatitis B virus (HBV) infection is estimated to affect more than 10% of the general population in Korea and liver cirrhosis and hepatocellular carcinoma related to chronic HBV infection are two of the leading causes of death.1)
Unfortunately, in spite of much effort to treat these patients, no treatment has been shown to alter the course or outcome of this disease. Corticosteroid therapy appears to have little or no effect in patients with chronic type B hepatitis. Some studies suggested that long-term and even short-term steroid therapy may be harmful in these patients.2–7) However, some other recent studies showed that short-term corticosteroid therapy followed by abrupt withdrawal may lead to an immune clearance of the virus with accompanying improvement.8,9) To investigate this possibility, we conducted short-term prednisolone therapy in patients with severe chronic type B hepatitis.
MATERIALS AND METHODS
1. Patients Studied
Ten patients with severe chronic type B hepatitis confirmed by liver biopsy who were positive for HBeAg were included in this study. Patients were comprised of 6 men and 4 women and ranged in age from 17 to 45 years (mean, 32 years) and were known to have had hepatitis B surface antigen (HBsAg) for longer than 6 months. Serum alanine aminotransferase (ALT) was elevated for a duration of more than one month before prednisolone therapy began. Histopathologically, all patients showed severe chronic type B hepatitis with confluent necrosis and/or acinar hepatitis.10) Prednisolone was administered 60 mg per day, and was tapered by 20 mg every two weeks for eight weeks. Patients were evaluated every 2 weeks during therapy and monthly thereafter for up to 15 months.
2. Serological Testing
The hepatitis B viral marker tests included HBsAg and antibody (anti-HBs), antibody to HBcAg (anti-HBc), and hepatitis Be antigen (HBeAg) and antibody (anti-HBe), which were assayed using a commercial radioimmunoassay (Ausria II, ausab, corzyme, HBeAg-Test: Abbott Laboratories, North Chicago, IL). Hepatitis B virus DNA (HBV-DNA) was measured by molecular hybridization using a radiolabeled HBV-DNA probe (donated by Dr. J. Gerin, Bethesda, MD) and was quantified by visual grading of the autoradiographs on a scale of 0 to 4+.
RESULTS
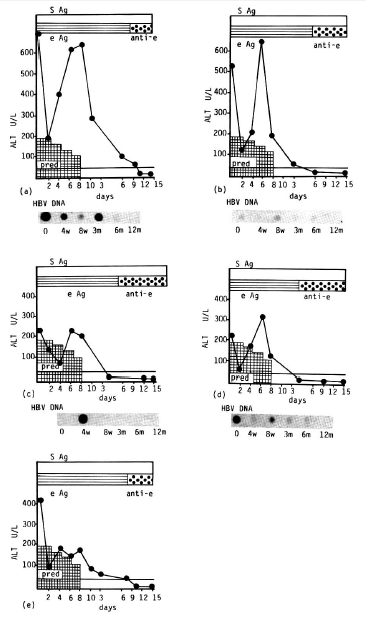
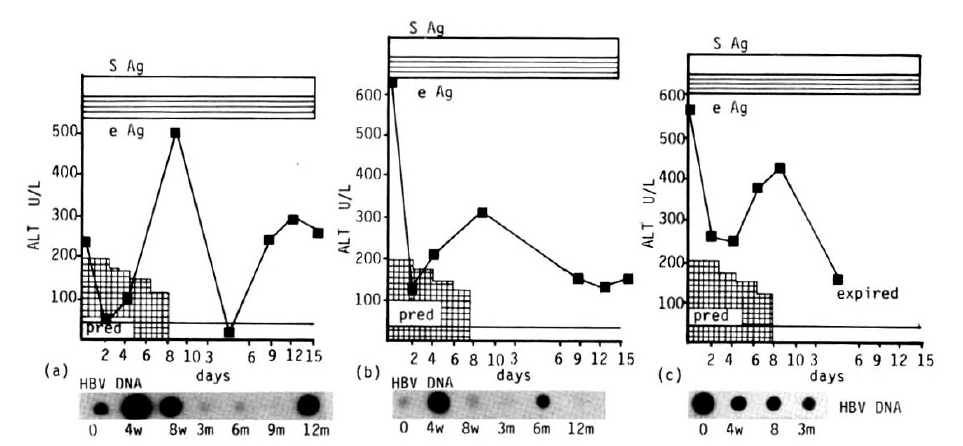
Of 10 patients treated with prednisolone, 5 were assigned to the responder group (those whose serum ALT returned to normal and who seroconverted from HBeAg to anti-HBe). The remaining 5 patients were assigned to the non-responder group (those whose serum ALT and HBeAg persisted). Initial clinical and biochemical characteristics of the patients studied were summarized in Table 1. Serum protein and albumin were normal and no patients showed jaundice, all 10 patients completed the 8 week course of therapy and have been followed for up to 15 months. Two to six months after cessation of therapy, 5 patients showed a disappearance of HBeAg and HBV-DNA and a return of serum ALT to normal (Fig. 1). In all 5 patients, the serum ALT level tended to decrease more than 50% of the initial level after two weeks of therapy and gradually returned to the pretreatment level. In all patients, a rebound of the serum ALT level was observed at the 4th and 8th week of therapy. However, the pattern of rebound was not consistent. Serum ALT was rebounded to nearly the same as the pretreatment level in 2 patients, more elevated than it in 2 and 50% of the initial level in 1. This pattern of variable change of serum ALT was also observed in 3 of 5 non-responders (Fig. 2). No rebound of serum ALT was observed during treatment in the remaining two patients. But no rebound of serum ALT was observed in any patient after the withdrawal of prednisolone. Among the five responders, serum HBV-DNA was detected in three patients initially and was transiently detected in one during therapy (Fig. 1). Serum HBV-DNA was not detected in one patient before and after treatment. In 4 patients who had detectable HBV-DNA in their serum initially and during therapy, no reappearance of HBV-DNA was observed after seroconversion of HBeAg to anti-HBe. In the 5 non-responders, HBeAg persisted during the follow-up periods and serum HBV-DNA persisted in three, but serum ALT decreased in all after cessation of prednisolone in comparison with the initial level, but did not return to normal. One patient, who did not show any clinical or serological improvement, died of jaundice, ascites and hepatic encephalopathy 4 months later (Fig, 2, case 3). By Southern blot analysis, the free form of HBV-DNA was observed in all patients, even though serum HBV-DNA was not detected in three patients. No integrated HBV-DNA was found. Side effects during prednisolone therapy included anxiety and nervousness in 3, increased appetite in all, facial sweling in 8, acne and other skin eruptions in 6. These side effects were subsided spontaneously after cessation of prednisolone.
DISCUSSION
Ten patients with severe chronic type B hepatitis were given prednisolone for an eight week period in an attempt to induce an immunologic clearance of hepatitis B virus and remission. In the present study, short-term prednisolone therapy did induce immunosuppression and tapering of the dosage did lead to transient increases in serum alanine aminotransferase activities and changes of serum HBV-DNA level. The rebound of enzymes did not lead to a permanent clearance of the virus, but did induce seroconversion of serum HBeAg to anti-HBe and return of serum ALT activity to normal during the follow-up in five of ten patients. The serum ALT level was markedly decreased in all patients with a high drug dosage but rebounded upon tapering of the dosage from the 3rd week of therapy.
In eight patients, the rebound of ALT was most striking while the dosage was tapered but no rebound of ALT was found after the withdrawal of therapy. Most of the other studies showed that the rebound phenomenon was not observed during therapy, but was observed after withdrawal of therapy.2–9) It may be related to the initial dosage of prednisolone or other unknown factors. Generally, spontaneous seroconversion of HBeAg to anti-HBe was reported to be preceded by an abrupt but transient elevation in serum ALT activity, which may be due to an increase in inflammatory activity in the liver.11) Furthermore, it is often suggested that acute exacerbation preceding spontaneous HBeAg clearance had resulted in severe hepatic decompensation with ascites, hepatic encephalopathy and even death.12) This sequence of events was closely parallel to our result. The mechanism of rebound of ALT change is not clearly understood. Some authors have postulated that an improvement in host immune status or “hyperimmune” condition after withdrawal of immunosuppressive therapy would mediate hepatic necrosis.13–16) Enhanced expression of viral antigens could modulate specific immune response after withdrawal of immunosuppressive agents. Hepatocyte membrane “core” antigen has been correlated with an enhanced level of T-lymphocyte cytotoxicity.17–20) Transient increase in HBV replication could expose infected hepatocytes with recognizable antigens on their surface and enhance their immune clearance when prednisolone is withdrawn. When prednisolone is rapidly withdrawn, one or more of these mechanisms may give rise to lysis of hepatocytes containing replicative forms of HBV-DNA with subsequent improvement in the serologic and biochemical parameters of active liver disease. In our study, we observed a replicative form of HBV-DNA in the liver by Southern blot analysis in all, even though serum HBV-DNA was not found in 3 patients (data not shown here).
Our results showed that 50% of the patients cleared HBeAg with return of serum ALT level to normal; however, at least three points should be emphasized. First, the short-term therapy with prednisolone might not be responsible for viral replication. Some studies showed no increase in HBV-DNA level during therapy,7) while some other data showed an increase in HBV replication markers during therapy.6,9,14) It should be noted that augmenting viral replication may induce seroconversion of HBeAg to anti-HBe. Second, the beneficial effect of therapy may be partly related to the severity of hepatitis activity. All of the histologic findings in our study, suggested that these patients may be severe enough to progress to the more aggressive form without therapy. It can be argued that patients with mild disease are unlikely to respond to any form of therapy21) and that inclusion of these patients may have negatively affected the results. However, short-term prednisolone therapy in patients with chronic active hepatitis with cirrhosis has confirmed that the rebound in disease activity after withdrawal can be accompanied by hepatic decompensation in the development of jaundice and ascites and even death. Finally, this is not a controlled study and only includes a small number of patients. Studies with larger numbers of patients and well designed control trials would be needed to evaluate the effect of prednisolone.
However, this study reaffirms the critical importance of the underlying histologic activity in the treatment of patients with chronic active hepatitis. Prednisolone therapy is not indicated in all types of chronic hepatitis. Careful selection based on clinical, biochemical, histologic and virologic criteria is required.