Effect of Adenine Arabinoside and α-Interferon in Patients with HBeAg-positive Chronic Active Hepatitis
Article information
Abstract
To evaluate and compare the therapeutic efficacy of adenine arabinoside (Ara-A) and alpha-interferon (α-INF), 40 patients with biopsy proven chronic active hepatitis B were chosen at random to receive Ara-A (15 mg/Kg, iv, for 10 day) or α-INF (3 million unit, sc, every other day for 12 wks) and followed up to 12 months after completion of the therapy. All patients were HBeAg positive. The clinical effects of Ara-A and α-INF on seroconversion of HBeAg positive. The clinical effects of Ara-A and α-INF on seroconversion of HBeAg and the levels of serum aminotransferase (ALT) were closely matched and compared with those of the untreated control group (20 cases). Eighteen out of 20 patients received Ara-A, 19 patients received α-INF, and 19 out of 20 control cases were evaluated at 12 months after completion of treatment.
Seroconversion of HBeAg in the α-INF treated group (19 cases) was observed in seven cases (36.8%), showing a higher seroconversion rate as compared to Ara-A-treated (2/18 cases, 11.1%) and to the control patients (1/19 cases, 5.3%).
There were no effects of Ara-A on serum ALT levels in the treated patients compared with the untreated control patients. However there was a remakale drop in serum ALT levels in the INF-treated patients (p<0.005, ALT levels at 12 months after treatment; 87.4±98.8 IU/L) compared to the pretreatment levels (256.7±175.8 IU/L).
The present study thus indicates that Ara-A is not recommended as the first choice of therapy in treating patients with HBeAg-positive CAH and that interferon may be recommended for those patients instead. Further study will be necessary with prolonged follow up.
INTRODUCTION
Chronic hepatitis B is an important cause of developing liver cirrhosis and hepatocellular carcinoma. Observations that seroconversion from HBeAg to anti-HBe is frequently associated with the normalization of liver function tests has encouraged the trial assessment of a number of antiviral agents in treating this disease, including adenine arabinoside1,2) adenine arabinoside monophosphate3,4) and interferons5–8). Though the collective data of most trials studies suggest that those antiviral agents appear capable of lowering HBV markers transiently, it is still unclear whether this exceeds spontaneous losses of viral markers. The present study describes the results of a prospective, randomized, controlled trial of adenine arabinoside (Ara-A) and natural α-interferon (INF) in light of seroconversion of HBeAg and the normalization of serum aminotransferase (ALT) in 40 patients with HBeAg-positive chronic active hepatitis B.
MATERIALS AND METHODS
1. Patients
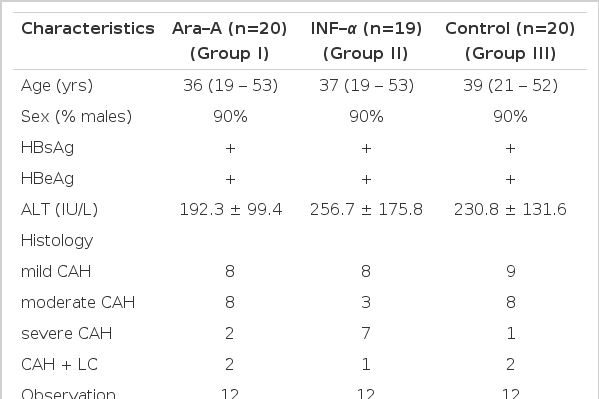
Sixty patients with documented chronic active hepatitis B participated in this study. Their pretreatment characteristics are shown in Table 1. All were known to have carried HBsAg for at least one year and to be positive for serum HBeAg. All patients had abnormal ALT and histologic evidence of chronic active hepatitis (CAH) on liver biopsy. No patients had received previous antiviral therapy nor any immunomodulatory agents. Excluded were patients with decompensated liver cirrhosis, organ transplants and malignant diseases. No patients were homosexual.
2. Study Design
At the start of the study, patients were admitted to the hospital and then allocated to one of three groups using a set of random numbers: Group I received Ara-A (Vidarabine, München, Germany) in a dose of 15 mg/Kg, iv. each day for 10 days; Group II received natural α-INF (Green Cross Company, Seoul, Korea) in a dose of 3 million units subcutaneously (sc) every other day for 12 wks; and Group III received no therapy.
3. Methods
Full blood counts and routine liver function tests were measured before the first dose of each treatment, at least one time every week during each course and monthly thereafter. The presence of HBsAg and anti-HBs were detected by enzyme immunoassay. HBeAg and anti-HBe were assayed by radioimmunoassay (Abbott Laboratories). Liver biopsy specimens obtained before entry were graded under code by a single observer for severity as either mild, moderate or severe CAH. Statistical analysis of laboratory data was done by paired Student’s t-test.
RESULTS
1. Patients
Of the 60 patients, 20 were assigned to Group I, 19 to Group II and 20 to Group III. All patients were followed up for 12 months after treatment. Two patients in Group I and one in Group III, who were treated with herb medicine during the follow-up period, were excluded from the final evaluation.
2. Effect on Serum HBeAg and HBsAg
In two out of the 18 treated patients with Ara-A, serum HBeAg became undetectable after 21 wks and anti-HBe subsequently developed within the next 20 wks. In contrast, nine out of the 19 patients in Group II had been HBeAg negative after eight months, and anti-HBe developed in seven cases within the next eight wks. Two cases who had been HBeAg negative became HBeAg-positive (retroconversion) at 12 months. All patients who achieved the development of anti-HBe did not become HBeAg postive. Among 19 control patients, only one achieved seroconversion of HBeAg at six months after randomization. These findings concerning the seroconversion of HBeAg were not changed at 12 months after completion of the therapy. No patients who entered in to this study lost HBsAg (Table 2).
3. Effect on Serum ALT Levels
Serum ALT levels in Group I patients, evaluated at six and 12 months, were not different from the pretreatment levels. In contrast, a significant decrease in ALT levels in Group II was observed after the fourth week of treatment, followed after therapy by an additional decrease up to 12 months. No such findings were observed in Group III (Table 3).
4. Side Effects
Ara-A and α-INF therapy used in the present study were well tolerated in all patients. Side effects observed in the Ara-A treated group were nausea and postprandial epigastric distress. The main symptom of α-INF were malaise, fever, myalgia, headache and nausea, all of which reversed after the completion of the therapy. Severe neutropenia and thrombocytopenia were not observed in the present study.
DISCUSSION
This study was done to evaluate whether a three-month course of α-INF therapy could be beneficial in inhibiting of viral replication and could improve liver function tests in a significant percentage of patients with HBeAg-positive CAH, as compared to Ara-A therapy in the control patients. In light of the seroconversion from HBeAg to anti-HBe and normalization of serum ALT activity, the results of our study suggested that a three-month course of α-INF therapy is beneficial for the treatment of chronic active hepatitis B.
Nevertheless, since the collective data of most trial studies have suggested that the effect of interferons in treating this disease was inconclusive9), a number of important factors essential in interpreting the responses to INF therapy should be considered. First, since the results of other workers who were treated with a short-term course of INF therapy5,6) showed less benefit than those under long-term therapy7,8), it may be possible that improving the schedule of administration of INF in a longterm course may be more beneficial in treating this disease. Second, it has to be considered that liver cells containing integrated HBV-DNA may not be eliminated by INF therapy10). Thus, the choice of patients who have the molecular tools to identify the presence or absence of integrated HBV-DNA in the liver cells may be important in predicting favorable responses to INF therapy. Finally, since the serum ALT level itself is not an absolute indicator of ongoing liver cell injury, it is necessary to follow the histological evolution of those responders and non-responders to INF therapy.
The toxidity of natural α-INF was a major problem in this study, and the side effects were both predictable and reversible.
In conclusion, the present study revealed that Ara-A is not recommended as the first choice of therapy in the treatment of patients with HBeAg-positive CAH, but natural α-INF in doses used in the present study may be recommended for those patients. However, long-term follow-up of INF-treated patients is required to determine whether INF therapy could reduce the risk of developing liver cirrhosis and primary hepatocellular carcinoma.