Changes in the Properties of the Thyrotropin Receptor Antibody in Patients with Graves’ Disease after Radioiodine Treatment
Article information
Abstract
We investigated the effect of a single dose of 131I upon thyrotropin receptor antibodies (TRAb) in 21 patients with Graves’ disease. The thyrotropin receptor antibodies were assessed by parallel measurements of thyrotropin binding inhibitor immunoglobulin (TBII), thyroid stimulating antibody (TSAb) and thyroid stimulation blocking antibody (TSBAb) in serum by radoreceptor assay, stimulation of adenlate cyclase and inhibition of TSH-stimulated adenylate cyclase activation in FRTL-5 cells, respectively. Prior to radioiodine treatment, TBII was detected in all 21 patients and TSAb in 19 patients. After radioiodine treatment, TBII activities did not change during the 12-months observation period, but in eight patients TSAb activities gradually decreased and were undetectable at the end of the 12-month observation period. Persistence of TSAb was not associated with the clinical outcome. Eight patients developed hypothyroidism within one year after radioiodine treatment. Three of the hypothyroid patients developed TSBAb, and the appearance of TSBAb coincided with the development of hypothyroidism. These results suggest that TSBAb might develop after radioiodine treatment in a minority of patients with Graves’ disease and that the appearance of TSBAb, in addition to radiation-induced thyroid destruction, might be involved in the development of hypothyroidism following radioiodine treatment.
INTRODUCTION
It is widely accepted that antibodies against the TSH receptor may play an important role in the cause of hyperthyroidism in Graves’ disease1). The activity of these antibodies can be estimated by direct biological activity, termed thyroid stimulating antibody (TSAb), or by their ability to inhibit the binding of radiolabeled thyrotropin to thyroid membranes, termed thyrotropin binding inhibitor immunoglobulins (TBII). It has recently been reported that immunoglobulins in primary nongoitrous myxedema not only inhibit the binding of TSH to its receptor, but also inhibit TSH-stimulated adenylate cyclase activation in cultured thyroid cells2,3), and these thyroid stimulation blocking antibodies (TSBAb) may play a role in the development of hypothyroidism and thyroid atrophy in primary nongoitrous myxedema4).
After radioiodine treatment for Graves’ hyperthyroidism, a transient increase, followed by a decline in TSH receptor antibodies (TRAb), has been well-documented5–9). Recently, it has been reported that TSBAb develop in patients with Graves’ disease during antithyroid drug treatment10) or after radioiodine treatment11,12). However, it is still uncertain whether changes in the properties of TRAb after radioiodine treatment, especially development of TSBAb, could alter the clinical outcome of Graves’ disease.
In the present study, we simultaneously meausred TBII, TSAb and TSBAb activities sequentially after a single dose of 131I and compared their activities with the functional status of the thyroid in patients with Graves’ disease.
MATERIALS AND METHODS
1. Patients
Twenty-one patients (10 male, 11 female), ranging in age from 15 to 72 years (mean age: 42 years), were studied. The diagnosis of Graves’ hyperthyroidism was based on clinical findings, elevated serum T4 level, decreased serum TSH level and increased thyroidal 131I uptake. No patients had been previously treated with 131I. Six of the patients received 131I as the first-line therapy, and the remainder were treated with antithyroid drugs for 12–70 months before 131I treatment.
Thyroid weight was estimated by palpation and scintiscan, and 100–150 uCi of 131I per gram of thyroid tissue was administered (4–15 mCi, mean±SD: 9.1±2.1 mCi). All patients were treated with methimazole for one or two months after the 131I administration, and if a relapse occurred, antithyroid medications were given until the completion of the study. After radioiodine treatment, blood samples were taken at three-month intervals up to 12 months. The IgG fraction was isolated from the serum samples by means of affinity chromatography on columns of protein A-Sepharose CL-4B (Pharmacia, Uppsala, Sweden). Thyroid function tests were done with commercially available RIA kits.
2. Assay for TBII
TBII was measured as previously described13) using a commercial radioreceptor assay kit (R.S.R. Ltd., Cardiff, Wales, UK). TBII activity was expressed as the percent inhibition of 125I-bTSH binding to the TSH receptor. A TBII value exceeding 20% was considered abnormal or positive.
3. Assay for TSAb and TSBAb
FRTL-5 cells, kindly supplied by Dr. L.D. Kohn (NIH, Bethesda, MD., USA), were maintained as previously described14) and also maintained for seven days in a medium without TSH before the assay. The medium was changed with 300 ul of test IgG (10 g/l). IgGs were dissolved in Hank’s balanced salt solution (HBSS) without NaCl containing 0.5 mmol/l IBMX, 20 mmol/l HEPES, and 1.0% BSA, pH 7.4. After 2 h incubation at 37°C, cAMP released into the medium was measured by RIA (Immunonuclear, Stillwater, Minn., USA). The assay system was sensitive to 5 mU/l bTSH with a response of 1.71±0.07 times the basal cAMP level. All samples were run in triplicate. The intrassay variance was 8.2–12.1%, and the interassay variance was 17.1–30.5%. TSAb activity was expressed as a % increase in cAMP production by test IgG in comparison with normal IgG. TSAb was defined as positive when the value was greater than 2 SD above the mean value produced by the IgG fraction from 24 normal subjects (>170%).
For the assay of TSBAb, test IgG was incubated with or without 0.1 U/l bTSH. Other procedures were the same as in the TSAb assay. TSBAb activity was expressed as a % inhibition of the TSH-stimulated cAMP increase and was calculated as follows: TSBAb (%) = 100 × [1 − (cAMP under 0.1 U/l bTSH with test IgG-cAMP under normal IgG)/(cAMP under 0.1 U/l bTSH with normal IgG-cAMP under normal IgG)]. TSBAb was defined as positive when the value was greater than 2 SD above the mean value produced by the IgG fractions from 24 normal subjects (>37%).
4. Statistical Analysis
Statistical analysis was done with a Fisher’s exact test and Wilcoxon rank sum test. Statistical significance was defined as having a P-value below 0.05.
RESULTS
Before radioiodine treatment, TBII was detected in all 21 patients, and TSAb was detected in 19 out of 21. The individual changes of TBII and TSAb activities after radioiodine treatment were variable. TBII activities increased in six patients, decreased in six and remained unchanged in nine; TSAb activities increased in four, decreased in 11 and did not change in four patients during the 12-month observation period (Table 1, Fig. 1). The changes of TBII and TSAb activities were not associated with pretreatment with antithyroid drugs (Table 1). At 12 months after radioiodine treatment, TBII was still present in all of the patients, but TSAb activity was not detected in eight patients, including the two in whom TSAb was undetectable before radioiodine treatment (Table 1). Between the patients whose TSAb activities persisted and those whose TSAb activities disappeared, there were no differences in age, sex, dose of 131I, duration of antithyroid drug treatment and titers of antimicrosomal and/or antithyroglobulin antibody. But TSAb activity before radioiodine treatment was significaltly higher in patients whose TSAb continued to persist than in those whose TSAb disappeared (Table 2).
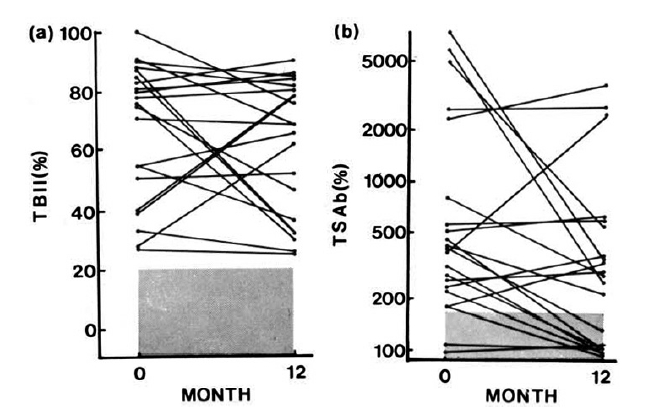
TBII (a) and TSAB (b) activities before and 12 months after radioiodine treatment in 21 patients with Gaves’ disease. The shaded area represents the normal range.

Initial Clinical and Laboratory Data (mean ± SD) in TSAb Non–disappearing and TSAb Disappearing Groups
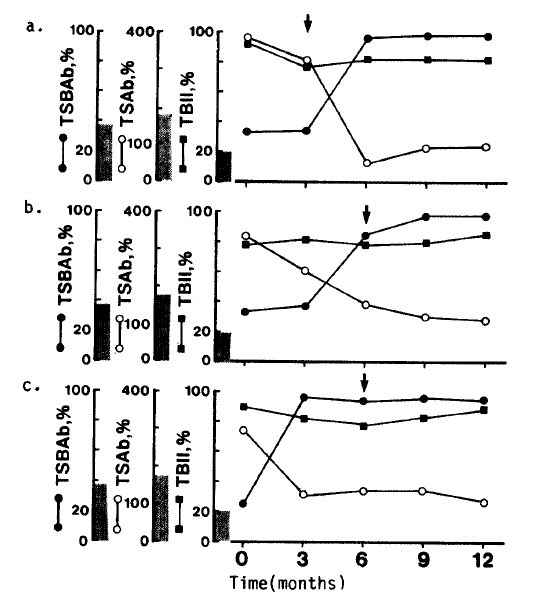
Twelve months after radioiodine treatment, eight patients had become hypothyroid, 10 were in remission and three had relapsed. The patients’ outcomes were not associated with TSAb activites at three and/or 12 months after radioiodine treatment. Pretreatment with antithyroid drugs did not affect the clinical outcome. Four out of eight patients who became hypothyroid were negative of TSAb activities. Of them, three patients had developed TSBAb during the observation peroid. All these patients became hypothyroid three to six months after radioiodine treatment, requiring thyroxine replacement (Table 3). Their TBII activities were potent before radioiodine administration and did not change after radioiodine treatment. But TSAb activities decreased gradually and disappeared within six months after radioiodine treatment, followed by a sudden appearance of TSBAb, coinciding with the development of hypothyroidism (Fig. 2). TSBAb activities were not found in any of the patients before radioiodine treatment.
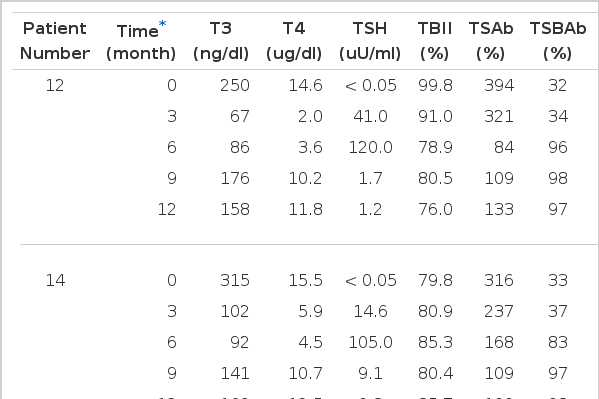
Serial Changes of Thyroid Function and TSH Receptor Antibodies Activities in Three Patients who Developed Hypothyroidism and Thyroid Stimulation Blocking Antibody After Radioiodine Treatment
DISCUSSION
In this study, we found that in contrast to previous reports8,9) TBII persisted for 12 months after radioiodine treatment in all patients. This discrepancy may be due to the differences in the patients selected and the short observation period. Most of the patients in this study were resistant to the long-term antithyroid drug treatment and had high TBII activities before radioiodine treatment. Moreover, TBII activities did not change or decrease at three months after radioiodine treatment in 15 out of 21 patients. In this study, all the patients were treated with antithyroid drugs for two or more months after radioiodine treatment. Their use after radioiodine treatment may have prevented a transient increase in TRAb activities. Changes of TSAb activities after radioiodine treatment varied. TSAb activities did not change for 12 months in 13 out of 21 patients. However, in six patients TSAb activities decreased gradually and then disappeared, while TBII persisted in high titers. This dissociation between TSAb and TBII activities suggests the development of different types of antibodies against the TSH receptor.
An interesting finding in this study is the development of TSBAb after radioiodine treatment in three out of 21 patients with Graves’ disease. All of them had both TBII and TSAb activities before radioiodine treatment. After radioiodine treatment, TSAb activities disappeared within six months and were followed by a sudden appearance of TSBAb activities. IgGs from these three patients inhibited not only the binding of TSH to its receptor but also a TSH-stimulated cAMP increase in FRTL-5 cells. Moreover, all of them developed hypothyroidism in accordance with the appearance of TSBAb. It is well-known that radioiodine therapy induces cell damage of the thyroid gland and results in hypothyroidism, but the present findings suggest that the appearance of TSBAb is in some cases related to the development of hypothyroidism.
The mechanism of changes in the properties of TRAb after radioiodine therapy in Graves’ disease is still unknown. Since we had used antithyroid drugs for several months before radioiodine treatment, the potential effects of antithyroid drugs on the properties of TRAb and their activities should be considered. However, we could not find any correlation between the pretreatment with antithyroid drugs and the clinical outcome after radioiodine treatment. It may be speculated that radioiodine not only causes a release of large amounts of antigens but also alters immune homeostasis in the thyroid gland, resulting in changes in the properties of TRAb. Although the incidence of the development of TSAb after radioiodine therapy is not frequent, out findings suggest that thyroid function after radioiodine treatment in Graves’ disease may be affected by changes in the properties of TRAb. Moreover, Furmaniak et al.15) recently described an interesting patient with Graves’ disease who developed TSBAb and hypothyroidism after radioiodine treatment, and TSBAb disappeared spontaneously with a reappearance of TSAb and hyperthyroidism. The clinical status of Graves’ disease might therefore be a result of the balance between the activities of stimulating and blocking types of TRAb16). Measuring several different actions of these TRAb might thus be of value in predicting the development of hypothyroidism in patients following radioiodine treatment.