Clinical features and prognostic factors of Churg-Strauss syndrome
Article information
Abstract
Background/Aims
Churg-Strauss syndrome (CSS) is a rare systemic necrotizing small-vessel vasculitis, with accompanying bronchial asthma, eosinophilia, and eosinophilic infiltration of various tissues. The purposes of our study were to characterize the clinical features of CSS and to identify factors associated with CSS prognosis in Koreans.
Methods
Medical records were reviewed retrospectively for all physician-diagnosed CSS patients in the Seoul National University Hospital between January 1990 and March 2011.
Results
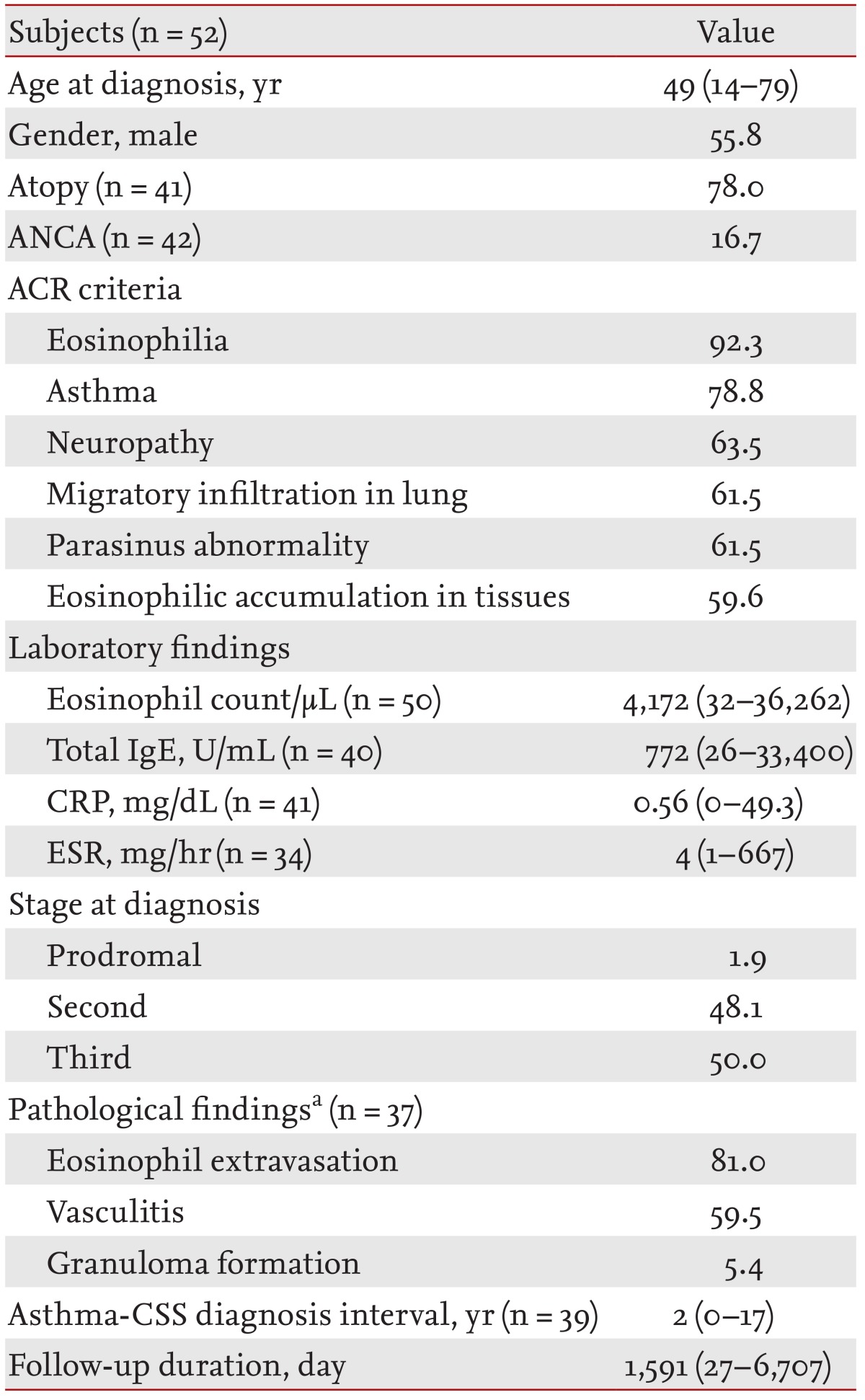
Data from 52 CSS patients were analyzed. The respiratory tract was the most commonly involved organ (90.4%). Renal involvement was less frequent in antineutrophilic cytoplasmic antibody (ANCA)(-) patients than in ANCA(+) patients (p = 0.048). Clinical remission occurred in 95.3% of patients, but 16.3% of them relapsed. Patients who maintained remission for more than 6 months were relatively older (median, 51 years) at diagnosis (p = 0.004), had been diagnosed in earlier stages (p = 0.027), showed more frequent respiratory involvement (p = 0.024) and generalized symptoms (p = 0.039), and showed less frequent cutaneous involvement (p = 0.030) than those who did not achieve persistent (> 6 months) remission. Patients who achieved persistent remission also showed higher C-reactive protein (CRP) levels (p = 0.031) than those who did not.
Conclusions
ANCA(-) CSS patients showed less frequent renal involvement. Characteristics of good responders were older age, diagnosis at earlier stages, less cutaneous involvement, more respiratory involvement, high CRP values, and more generalized symptoms.
INTRODUCTION
Churg-Strauss syndrome (CSS) is a rare systemic necrotizing vasculitis, involving small-medium vessels. Characteristic features of CSS include hypereosinophilia, eosinophil infiltration in various tissues, and granuloma formation [1]. The syndrome was f irst named allergic angiitis and granulomatosis in 1951 by Churg and Strauss [2]. They described 13 autopsy cases presenting with fever, hypereosinophilia, and evidence of vasculitis and identified the disorder as a disease that was distinguishable from other systemic vasculitides [2].
The CSS prevalence is highest at a mean age of 48, with an equal gender distribution, although the syndrome can occur at any age [3]. Although the annual incidence is low in the general population, 2.4 to 13 per million persons, it is relatively high in asthma patients, 34.6 to 64.6 per million persons [4,5]. It is not easy to diagnose CSS because of its low incidence and the variety of clinical features at each the stage. Additionally, the definite causes and pathophysiology of CSS remain not well understood [5].
Antineutrophilic cytoplasmic antibodies (ANCAs) can be detected in 40% of CSS patients, and some investigators have hypothesized that there are two types of CSS, based on the presence (+) or absence (-) of ANCA [6-8]. In ANCA(+) patients, renal involvement, neuropathy, alveolar hemorrhage, and vasculitis with purpura are predominant, while in ANCA(-) patients, cardiac and pulmonary involvement are predominant [6-8]. However, the two types are in many cases not distinct, and there is no evidence at this stage indicating that the two types are associated with different prognoses or should be treated differently [6-8]. Thus, the presence or absence of ANCA does not affect therapeutic plans [6-8].
Previous studies on CSS patients in Korea are mostly case reports [9-16]. In 2000, two clinical reports were published: one on intravenous high-dose cyclophosphamide infusion in five patients who had neuropathy refractory to a high-dose steroid, and the other on the electrophysiological features of the peripheral neuropathy in 12 CSS patients [17,18]. In 2006, a third report of the clinical features and long-term follow-up results of 17 CSS patients was published [19].
Here, we analyze the clinical features, responsiveness to treatment, factors associated with ANCA status, and responsiveness to treatment in 52 patients with CSS, a relatively large number compared with previous studies in Korea.
METHODS
All patients who had been diagnosed with CSS by a clinician at the Outpatient Department of Seoul National University Hospital during the period January 1990 to March 2011 were enrolled. We retrospectively searched their electronic medical records and reviewed the age at diagnosis, gender, and atopy of each patient. To confirm the presence of atopy, a skin prick test was performed using an inhaled allergen comprising 55 items. Atopy was defined as a positive result for more than one item [20].
ANCA levels can be measured using indirect immunofluorescence assays or enzyme immunoassays (EIAs) [21]. Proteinase III (Pr III) is a specific antigen for cytoplasmic-staining-pattern ANCA (c-ANCA), while myeloperoxidase (MPO), elastase and lactoferrin are specific antigens for perinuclear-staining-pattern ANCA (p-ANCA) [22]. In our study, Pr III Ab and MPO Ab were measured using EIAs. If one of those results was above 20 units, the result was deemed positive. We also determined patient eosinophil counts in peripheral blood, immunoglobulin E (IgE), and C-reactive protein (CRP) levels, and the erythrocyte sedimentation rate.
To evaluate the clinical features of CSS patients, we reviewed the patient's classification using the six CSS criteria proposed by The American College of Rheumatology (ACR): eosinophilia, asthma, neuropathy, migratory infiltration in the lung, parasinus abnormality, and eosinophilic accumulation in tissues [23]. If a patient satisfies four of the six criteria, the sensitivity is 85%, and the specificity is 99.7% [23]. The stage at diagnosis, pathological f indings of tissue specimens, asthma-CSS diagnosis interval, follow-up duration, organ involvement, generalized symptoms, treatment, duration and dose of steroid administration, and response to treatment were also reviewed.
Remission was defined as symptom improvement, normalization of the eosinophil count in peripheral blood, and absence of clinical evidence of active vasculitis. Patients who were observed for more than 6 months were classified into two groups: good responders were patients who achieved persistent remission (> 6 months) without relapse, and poor responders were patients who did not [5].
The natural course of CSS consists usually of three phases. The first, prodromal stage is characterized by allergic rhinitis and accompanying asthma, sinusitis, and nasal polyposis. The second stage is characterized by peripheral blood eosinophilia with eosinophil tissue infiltration. In the third stage, vasculitis prevails [4].
Statistical analyses were conducted using the Mann-Whitney U test and Fisher exact test for comparisons between groups: ANCA(+) versus ANCA(-) patients, and good versus poor responders. These analyses were performed using the SPSS software version 18.0 (IBM Co., Armonk, NY, USA). Results were considered statistically significant when two-sided probability p values were less than 0.05.
RESULTS
Clinical characteristics of Churg-Strauss syndrome
In total, 52 patients were enrolled. Their baseline characteristics are listed in Table 1. The group's median age was 49 years (range, 14 to 79), and the gender distribution was approximately equal (males, 55.8%). Most patients (78%) showed atopy in skin prick test results. ANCA was measured in 42 patients and was positive in seven (16.7%). Five patients had taken a leukotriene antagonist to control asthma symptoms.
All patients were categorized according to the six ACR criteria. Of the 52 patients, 20% satisfied all six ACR criteria, 26.9% satisfied five, 38.5% satisfied four, 15.4% satisfied three, and 7.7% satisfied two criteria. In total, 76.9% of these patients satisfied at least four of the ACR criteria. Twelve patients who did not satisfy at least four criteria were diagnosed with suspicious CSS by allergy, rheumatology, and neurology specialists. Among the ACR criteria, eosinophilia was the most commonly satisfied criterion (92.3%), followed by asthma, neuropathy, migrating infiltration in lung, parasinus abnormality, and eosinophilic accumulation in tissues, in that order (Table 1).
Most patients (98.1%) were diagnosed at the second and third stage of CSS; only one patient (1.9%) was diagnosed to be at the prodromal stage (Table 1).
In total, 47 biopsies were performed in 37 patients. The most common biopsy sites were skin (19 biopsies) and nerve (11 biopsies). Other sites were the gastrointestinal tract (six biopsies), nasopharynx (three biopsies), lung parenchyma (three biopsies), kidney (three biopsies), and myocardium (two biopsies). Among the biopsied patients, eosinophil extravasation was the most common pathological finding (81%), followed by vasculitis (59.5%) and granuloma formation (5.4%) (Table 1). Of those patients, 43.2% concurrently exhibited eosinophil extravasation and vasculitis.
Among the 41 patients with asthma, there was a median of 2 years (range, 0 to 17) of disease duration prior to CSS being diagnosed (Table 1). Six of those patients were diagnosed with asthma at the time of their CSS diagnosis. The median follow-up duration for all patients was 1,591 days (range, 27 to 6,707) (Table 1).
Organ involvement in Churg-Strauss syndrome
The frequencies of organ involvement in CSS patients are listed in Table 2. The respiratory tract (90.4%) was the most commonly involved organ, followed by neurological, cutaneous, gastrointestinal, renal, and cardiac system organs, in that order.
Asthma (78.8%) was most common feature of respiratory involvement, with sinusitis (61.5%) the next most common. All patients had a simple chest X-ray image taken and 34 patients (65.4%) showed normal findings on those images. Patchy consolidation (11 cases, 21.2%) was the most common abnormal finding on the chest X-ray images; pleural effusion (four cases), peribronchial infiltration (one case), nodules (one case), and emphysema (one case) were also observed. Computed tomography (CT) was performed in 38 patients, and abnormal findings were seen in 29 patients (76.3%). Among the abnormal f inding, 16 patients showed ground-glass opacification while others showed nodules (eight cases), bronchial wall thickening (seven cases), and pleural effusion (three cases).
Nervous system involvement was observed in 32 patients. Peripheral nervous system involvement was predominant (29 cases) versus central nervous system involvement (four cases). The central nervous system cases included cerebral infarct with vasculitis (three cases) and diplopia due to the fourth central nerve palsy (one case). One patient had concurrent peripheral and central nervous system involvement.
Electromyography/nerve conduction studies (EMG/NCS) were performed in 25 patients. Axonal type mononeuropathy multiplex was observed in 10 of those patients (40%), and sensorimotor polyneuropathy was observed in a further nine patients (36%). The remaining EMG/NCS patients had nonspecif ic neuropathy (three patients, 12%), radiculopathy (two patients, 8%), and one patient had normal EMG/NCS findings.
Cutaneous involvement was observed in 23 patients, with the most common presentation being nonpalpable purpura (seven patients). Urticaria and rash (six patients), erythematic rash (five patients), subcutaneous nodules (two patients), and dermatitis (one patient) were also observed. The remaining two patients indicated that they had cutaneous involvement, but there was no definite description of their involvement types.
Gastrointestinal involvement was observed in 16 patients, with abdominal pain being most common (10 patients). Anorexia (three patients), nausea and vomiting (two patients), diarrhea (two patients), bowel perforation (one case), and ulcer (one case) were also observed.
Cardiac involvement was observed in six patients. Right bundle branch block (three cases), pericardial effusion (two cases), regional wall motion abnormality (one case), and diastolic dysfunction (one case) were also observed. Myocardial biopsies were performed in the patients with regional wall motion abnormality and diastolic dysfunction; each pathological specimen showed subepithelial and myocardial fibrosis and eosinophil infiltration.
Renal involvement was observed in six patients, with microscopic hematuria (five patients) and proteinuria lower than 1 g/24 hours (three patients) being observed.
Generalized symptoms were reported in 35% of patients, and presented as general weakness, body weight loss, febrile sense, and myalgia.
Treatment and clinical progression of Churg-Strauss syndrome
Treatments and responses to treatment are summarized in Table 3. No subject died. All 52 patients received a systemic steroid, and some patients (34.6%) received additional immunosuppressants (cyclophosphamide or azathioprine). The initial prednisolone loading dose was 0.9 mg/kg (range, 0.3 to 17.6). The median maintenance dose of prednisolone was 0.1 mg/kg (range, 0.04 to 1.12). The median duration of steroid administration among 38 patients was 1,495 days (range, 33 to 6,726).
Comparison of clinical features according to ANCA status
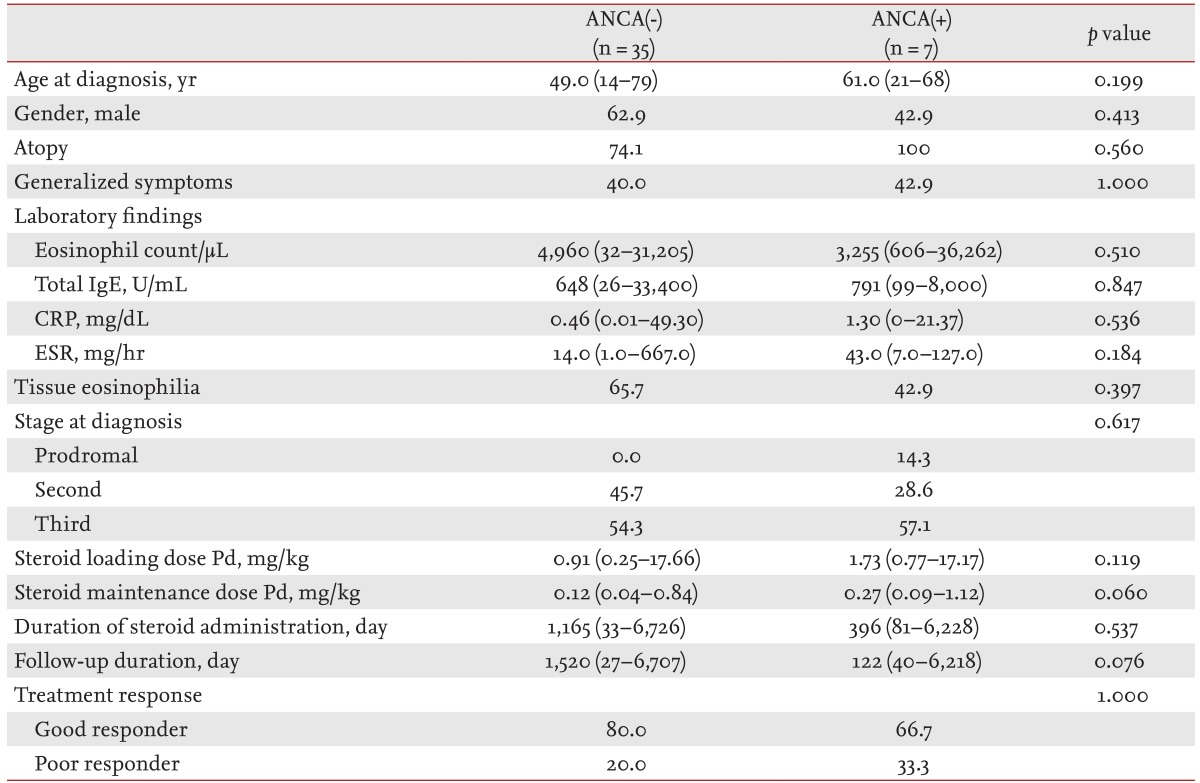
There was no difference between ANCA(-) and ANCA(+) patients in epidemiology, laboratory findings, stage at diagnosis, initial loading dose, duration of steroid administration, or response to treatment (Table 4). ANCA(-) patients received a lower median maintenance dose of steroid and were followed-up for a longer median duration than ANCA(+) patients; however, the differences were not statistically significant. Time to remission was longer in ANCA(+) patents than ANCA(-), although the difference was not statistically significant (12.5 months [range, 12 to 13] vs. 6 months [range, 1 to 27]; p = 0.227).
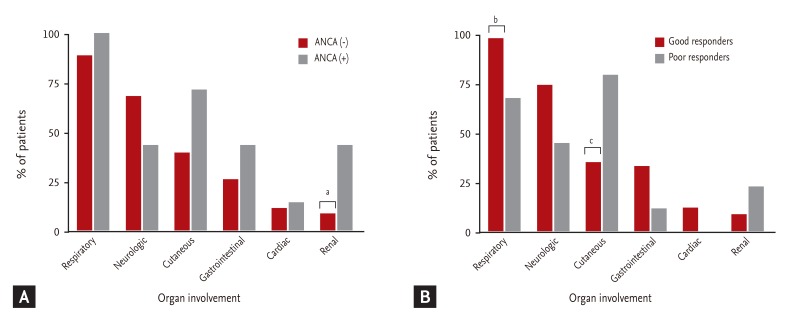
Differences in organ involvement according to ANCA status are presented in Fig. 1A. Renal involvement was significantly less frequent in ANCA(-) than in ANCA(+) patients (8.6% vs. 42.9%; p = 0.048). In ANCA(-) patients, cutaneous and gastrointestinal system involvement were less frequent (40.0% vs. 71.4% and 25.7% vs. 42.9%, respectively), and neurological involvement was more frequent than in ANCA(+) (68.7% vs. 42.9%); however, the differences were not statistically significant.
Comparison of clinical features according to treatment response
Of the 43 patients who were observed for more than 6 months, 41 patients (95.3%) achieved remission, and two patients (4.7%) were refractory to treatment (both men). There was no case of mortality. Of the two refractory patients, one was diagnosed at 14 years old and had peripheral blood eosinophilia, asthma, peripheral neuropathy, parasinus abnormality, ground glass opacity on CT, subcutaneous nodules on skin biopsy, positive results for ANCA, elevated IgE (8,000 U/mL), and abdominal pain. He had taken oral prednisolone for 5 years but his abdominal pain did not resolve. The other patient was diagnosed at 66 years and had peripheral eosinophilia, peripheral neuropathy, parasinus abnormality, patch consolidation on chest X-ray, and abdominal pain. He did not respond to systemic steroids and also took cyclophosphamide. After that, he was lost to follow-up.
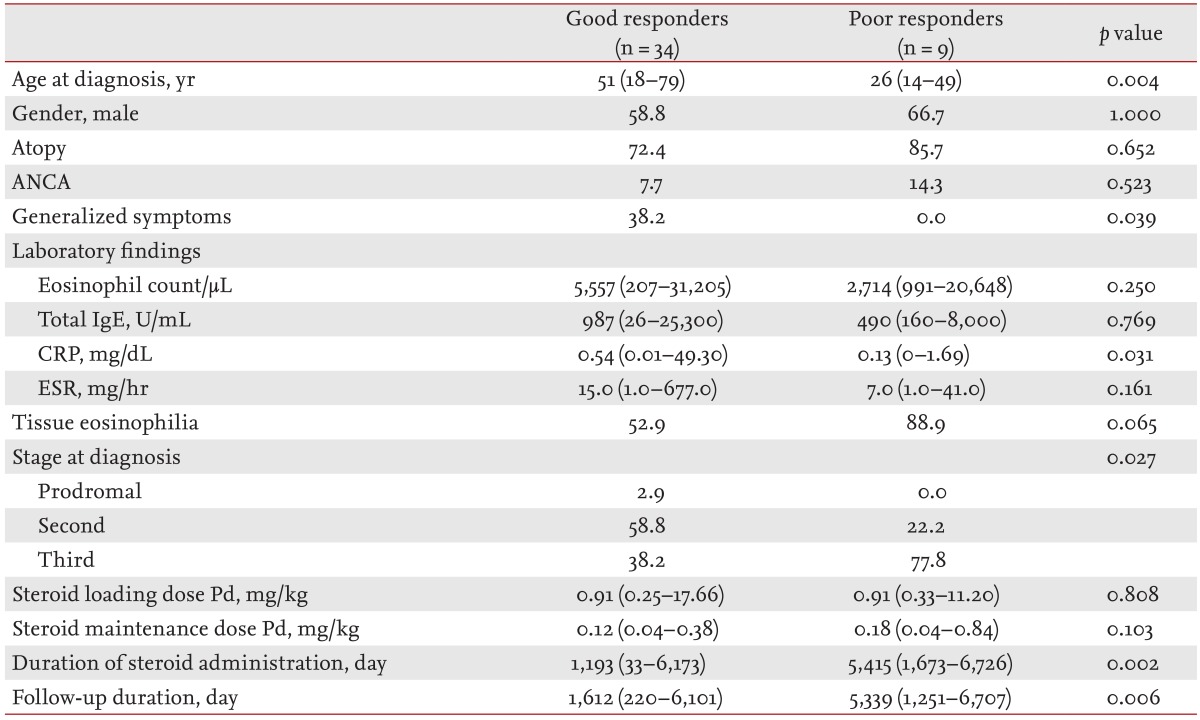
Of the 43 patients who were observed for more than 6 months, 34 (79.1%) were classified as good responders, while the remaining nine (20.9%) were poor responders. A comparison of the clinical features of good and poor responders is shown in Table 5. The median age of CSS onset was significantly higher in good responders (51 years) than in poor responders (26 years; p = 0.004). In good responders, generalized symptoms and CRP values were significantly higher than in poor responders (p = 0.039 and p = 0.031, respectively). Tissue eosinophil infiltration was observed in 52.9% of good responders and in 88.9% of poor responders; however, the difference was not statistically significant. In good responders, 58.8% of patients were diagnosed at the second stage of CSS, but in poor responders, 77.8% were diagnosed at the third stage, a significant difference (p = 0.027). Initial loading and maintenance doses of steroid did not differ significantly between the groups. However, good responders received steroid treatment for a shorter period (p = 0.002) and were followed up for a shorter period (p = 0.006).
Differences in organ involvement, based on treatment response, are shown in Fig. 1B. Among the good responders, respiratory involvement (97.1% vs. 66.7%; p = 0.024) was observed more frequently, and cutaneous involvement less frequently (35.3% vs. 77.8%; p = 0.030) than in poor responders. Renal involvement was observed less frequently in good responders (8.8% vs. 22.2%), but the difference was not significant.
Time to remission was longer in patients who experienced relapse than good responders with a significant difference (18 months [range, 2 to 29] vs. 5.5 months [range, 1 to 19]; p = 0.013).
DISCUSSION
In this study, we analyzed all CSS patients who were diagnosed over a 22-year period at a single tertiary hospital. Although several diagnostic criteria for CSS have been proposed, standardized criteria have not yet been established. One such diagnostic approach is to use the ACR classification criteria. In this study, 23.1% of patients did not satisfy the ACR criteria. However, they were diagnosed as suspicious for CSS by their clinician and were managed based on treatment for CSS. All showed improvement of symptoms, and additional findings did not result in the clinician doubting the CSS diagnosis.
Asthma is typically seen in the prodromal stage of CSS, and was reported in 96% to 100% of CSS patients [8,19]. In 2006, there was a report in which all 17 CSS patients also had asthma [8,19]. However, in our study, 41 of 52 patients (78.8%) were diagnosed with asthma, lower than in previous studies. Among them, five patients were taking a leukotriene antagonist at CSS diagnosis. In some studies, a leukotriene antagonist has been suggested to affect the pathogenesis of CSS [1]. However, we did not analyze whether it could be a risk factor for CSS because too few cases were available.
Also, five of 11 patients who were not diagnosed with asthma did show bronchial hypersensitivity in methacholine provocation test results (PC20 < 16 mg/mL). If these five patients were classified as potential asthma patients, the asthma prevalence in our study would increase to 86.5%. As data were lacking, we could not determine whether the six remaining patients had bronchial hypersensitivity; thus, we could not exclude the possibility. This suggests the need to examine all CSS patients who may have potential asthma. In our study, neurological and cutaneous involvement were the most common extrapulmonary manifestations. These results are consistent with previous reports [19].
Some researchers have suggested that clinical assessment of ANCA status is useful as a CSS presentation, and some hypotheses regarding its pathophysiology have been proposed [6,7]. First, the presence of ANCA could activate neutrophils, which, in turn, could produce reactive oxygen species and release lysosomal proteolytic enzymes that are included in neutrophil granules [24]. Second, the presence of ANCA could increase vascular permeability and inf luence vascular endothelial cells, effects that have been shown in murine models [25,26]. Some investigators insist that two types of clinical features are related to the presence of ANCA, and that vasculitis is predominant in ANCA(+) patients [6,7]. However, consistency in the clinical features of CSS, such as eosinophilia and tissue eosinophilic accumulation, has not been reported, so correlations between ANCA status and clinical features are not well founded. In our study, ANCA(-) patients showed significantly less renal involvement, less frequent cutaneous involvement, and more neurological involvement than ANCA(+) patients. Moreover, peripheral eosinophil counts were higher in ANCA(-) patients. Based on these f indings, different pathophysiologies related to the presence of ANCA could exist in CSS.
The prognosis of CSS is reportedly better than that for other types of vasculitis [8]. Before steroid treatment was used routinely in CSS, the mortality rate in the 3 months after diagnosis was as high as 50% [8]. However in a study of treatment with steroid and immunosuppressants in 2005, it was reported that the 5-year survival rate was up to 98% [27,28]. In our study, no subject died. However, not all patients had the same prognosis. Stage of diagnosis and organ involvement features have been reported to inf luence CSS prognosis [29]. Five factors-cardiomyopathy, severe gastrointestinal involvement, central nervous system involvement, 24-hour urine protein over 1 g, and serum creatinine over 1.4 mmol/L-have been associated with CSS prognosis. These factors have been used to develop the so called five-factor score (FFS), which can be used to help decide on a therapeutic plan [30]. Although the validity of the FFS has not been demonstrated, a 2011 revision to FFS added two more factors: age over 65 years and involvement of ear, nose, and throat. Additionally, the serum creatinine level was adjusted from 1.4 to 1.5 mmol/L, and central nervous system involvement was excluded [31]. In our study, we evaluated the FFS of all 52 patients at diagnosis, and 45 of those patients (86.5%) had an FFS of zero. The remaining seven patients (13.5%) had an FFS of one.
Between good and poor responders, organ involvement was different. Good responders had higher respiratory involvement and lower cutaneous involvement than poor responders. Additionally, older age, earlier diagnosis, and higher CRP levels were associated with a good response to treatment. Although the 2011 revised FFS included age over 65 years as a factor, in our study, there were more older patients among good responders than among poor responders. A possible explanation is that older patients might have more comorbidities, and have an increased probability of visiting a hospital. This could influence compliance in older patients. We compared relapse rates between patients above and below the age of 65 years (data not shown). In patients aged below 65 years, seven of 36 patients (19.4%) experienced relapse, but in patients aged over 65 years, none of the four patients experienced relapse. However, there was no statistically significant difference between the groups. Because there were only four patients in the age over 65 years group, it is difficult to conclude that older age is associated with a good CSS prognosis. Further studies with larger sample sizes are clearly needed to determine whether the age-related difference in CSS prognosis is limited to our study group or is a specific feature of Korean CSS patients.
One result of particular note is that the stage at CSS diagnosis correlated with the response to treatment. In good responders, 59% of patients were diagnosed at the second stage and 38% of patients were diagnosed at the third stage. However, in poor responders, 78% of patients were diagnosed at the third stage, a significant difference. Vasculitis is a major cause of death in CSS patients and a delay in diagnosis until the third stage could make the progression of disease a negative factor, and could be related to the relapse rate [3].
A final factor reported to be associated with CSS prognosis is the CRP level. In our good responders, the median CRP value was higher than in our poor responders. Most CSS patients experience an elevation in CRP levels [6], but we cannot offer a definite explanation as to why a high CRP level is associated with a good response. No previous study has reported an association between high CRP level and good response to CSS treatment. Further studies are needed to identify and explain factors associated with CRP levels and CSS prognosis.
The medical treatment was not specified according to patient medical condition [1]. In our study, there was no significant difference in relapse, steroid dose, or duration in the medical treatment groups (Supplementary Tables 1 and 2 online). There were limitations to assessing the medical condition of each patient because of the information in the electronic medical records. Further studies are needed to obtain evidence as to which medical treatments would be appropriate.
Our retrospective study had some limitations. First, the follow-up durations differed. Additionally, patients who did not have a sufficient follow-up duration were not evaluated for response status, whether they experienced relapse or not. The relapse rate reported in a previous study was 25% [3], whereas our relapse rate (17.3%) was lower. To address this, additional information about the 11 patients who did not follow-up would be needed. Second, some initial information on our patients was insufficient because we included patients who were referred from outside hospitals; thus, the exact steroid loading and/or maintenance doses in four patients were unknown. Third, whether some clinical findings are actually associated with CSS is difficult to establish. We could not compare these findings after diagnosis with those before diagnosis in most patients. Our data showed only the epidemiological findings in CSS patients at diagnosis.
Despite these limitations, we present additional information on the clinical features of Korean CSS patients, a population in which the CSS prevalence is low. This report could be of particular clinical significance because it is the first to enroll a relatively large number of Korean patients and to analyze clinical differences according to ANCA status and other associated factors related to the response to CSS treatment in Koreans.
In conclusion, we analyzed the clinical features in 52 CSS patients who visited a single tertiary hospital. The presence of ANCA could affect the clinical presentation with renal involvement. Several factors, such as age and stage at diagnosis, organ involvement, and CRP levels, were associated with the response to treatments.
KEY MESSAGE
1. The presence of antineutrophilic cytoplasmic antibody could affect the clinical presentation with renal involvement in Korean patients.
2. Characteristics of good responders were older age, diagnosis at earlier stages, less cutaneous involvement, more respiratory involvement, high C-reactive protein level, and more generalized symptoms.
Acknowledgments
We thank all the members of the Institute of Allergy and Clinical Immunology at Seoul National University Medical Research Center. There was no financial support.
Notes
No potential conflict of interest relevant to this article is reported.