Validation and reliability of a Behcet’s Syndrome Activity Scale in Korea
Article information
Abstract
Background/Aims:
We prepared a cross-cultural adaptation of the Behcet’s Syndrome Activity Scale (BSAS) and evaluated its reliability and validity in Korea.
Methods:
Fifty patients with Behcet’s disease (BD) who attended the Rheumatology Clinic of Gachon University Gil Medical Center were included in this study. The first BSAS questionnaire was administered at each clinic visit, and the second questionnaire was completed at home within 24 hours of the visit. A Behcet’s Disease Current Activity Form (BDCAF) and a Behcet’s Disease Quality of Life (BDQOL) form were also given to patients. The test-retest reliability was analyzed by intraclass correlation coefficients (ICC). To assess the validity, the total BSAS score was compared with the BDCAF score, the patient/physician global assessment, and the BDQOL by Spearman rank correlation.
Results:
Twelve males and 38 females were enrolled. The mean age was 48.5 years and the mean disease duration was 6.7 years. Thirty-eight patients (76.0%) returned the questionnaire by mail. For the test-retest reliability, the two assessments were significantly correlated on all 10 items of the BSAS questionnaire (p < 0.05) and the total BSAS score (ICC, 0.925; p < 0.001). The total BSAS score was statistically correlated with the BDQOL, BDCAF, and patient/physician global assessment (p < 0.01).
Conclusions:
The Korean version of BSAS is a reliable and valid instrument to measure BD activity.
INTRODUCTION
Behcet’s disease (BD) is a chronic, multisystem disorder [1,2]. We validated the Behcet’s Disease Current Activity Form (BDCAF, from the revised version, 2006) in Korea to evaluate BD activity [3,4]. It is difficult to evaluate the BDCAF during busy clinical work, because it must be administered by a clinician. However, it is necessary to evaluate how useful a patient-derived questionnaire would be in comparison. Forbees et al. [5] developed the Behcet’s Syndrome Activity Score (BSAS) as a self-reported patient form, which has since been adapted for use in other countries [6]. The aim of this study was to create a cross-cultural adaptation of the BSAS in the Korean language and to evaluate its reliability and validity in Korean patients with BD.
METHODS
Patients
Patients with BD were recruited at the Rheumatology Clinic of Gachon University Gil Medical Center. Data collection from patients took place from July to December 2013. All patients were over 18 years of age and fulfilled the criteria of the International Study Group for Behcet’s Disease [7]. All patients gave written informed consent to participate in this study, and the Institutional Review Board of Gachon University Gil Hospital approved the study protocol.
BSAS questionnaire
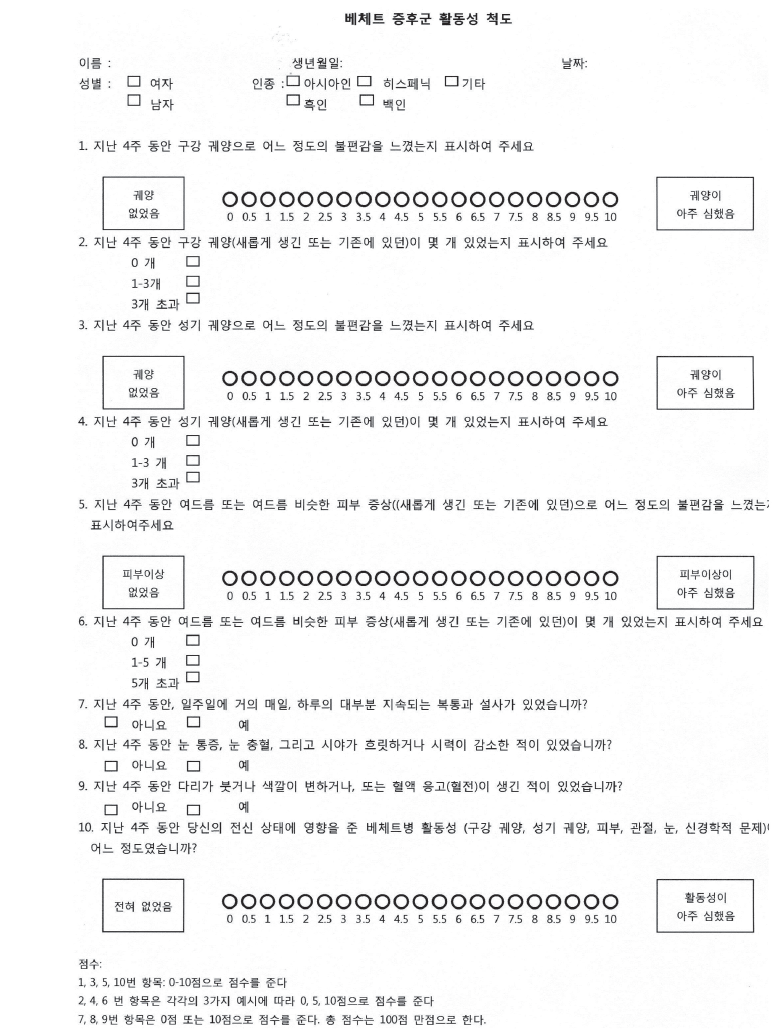
The BSAS score comprises 10 items. Questions about oral ulcers (q1), genital ulcers (q3), skin lesions (q5), and current disease activity (q10) are scored from 0 to 10 (visual analogue scale [VAS]), questions about the number of oral ulcers (q2), genital ulcers (q4), and skin lesions (q6) were scored as 0, 5, or 10, depending on which of the three were checked (0, n = 0; 5, n = 1 to 3; 10, n > 3 for q2 and q4; and n > 5 for q6), and questions about gastrointestinal/eye/vascular symptoms (q7, q8, and q9) were scored as 0 or 10, depending on whether or not they were present. The total score possible is 100.
Cross-cultural adaptation and survey method
The cross-cultural adaptation followed the guidelines proposed by Beaton et al. [8], after permission was obtained by the developer to use the BSAS. Two translators who were aware of the objectives of the study performed a primary translation into the Korean language. The questionnaire was then back-translated into English by two English teachers (native speakers), acting as independent translators who were unaware of the study objectives. The investigators discussed the discrepancies between the translation and the back-translation of the questionnaire before it was finalized (Appendix 1).
The first BSAS questionnaire was administered to patients during their initial visit to the rheumatology clinic (time 1) and was collected on the same day. The second BSAS questionnaire was scored by the patients at home 1 day after the first questionnaire (time 2) and was sent to us via mail. The questionnaire took 5 to 10 minutes to complete. At the first assessment, the BDCAF [4] and the clinician’s overall perception of disease activity over the 4 weeks prior (via VAS; consisting of seven different facial expressions and ranked on a scale of 1 to 7) were recorded, and the patients were asked to assess their overall perception of disease activity (patient’s VAS; range, 1 to 7). The BDCAF total score was calculated out of 12 and then given as a transformed index score on an interval scale. At each BSAS assessment, a Korean version of Behcet's Disease Quality of Life (BDQOL-K) form [9], containing 30 questions about quality of life (score range, 0 to 30), was also completed by the patients.
Statistical analysis
Agreement between the first and second assessments was assessed by calculating the intraclass correlation coefficients (ICC). The agreement between the BSAS score and the BDCAF and the patient’s/physician’s VAS and the BDQOL were calculated by Spearman’s correlation method. A correlation coefficient of less than 0.3 was considered low, 0.3 to 0.6 was moderate, and > 0.6 was high. Null hypotheses of no difference were rejected if the p values were < 0.05. Statistical analysis was performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Clinical characteristics of patients
Fifty patients were enrolled, and 76% (n = 38) were female. The mean age was 48.5 years, and the mean disease duration was 6.7 years. All patients (100%) had oral ulcers. Pathergy test positivity was 61.5%, and HLA-B51 positivity was 46.2% (Table 1). The most common occupation was housewife (40.0%), and 39 patients (78.0%) had a spouse. Most patients had 9 to 12 years of education (38.0%).
Test-retest reliability and validity of BSAS
The agreement between BSAS items 1 and 2 is shown in Table 2. Thirty-eight patients (76.0%) returned their questionnaires by mail. Nine out of 10 items correlated well (ICC, 0.666 to 0.924; p < 0.001). There was moderate agreement in one item concerning gastrointestinal involvement (ICC, 0.481; p = 0.025). The test-retest reliability of the total BSAS score was also good, with a high correlation between the time points (ICC, 0.925; p < 0.001). Bland-Altman plots were created to confirm the reliability. The mean difference between BSAS 1 and 2 scores was −1.66; the upper and lower limits were 19.39 and −22.71 (Fig. 1), respectively. Linear regression analyses between the differences and the means of the two BSAS scores also showed no statistical significance. The mean total BSAS score was 27.4 at time 1, which decreased to 26.0 at time 2 (Table 2). The mean BDQOL score was 8.6, which decreased to 8.1 at time 2; the mean patient VAS was 3.8, and that of the physician was 2.9, and the mean transformed BDCAF score was 7.4 at the first assessment (data not shown).
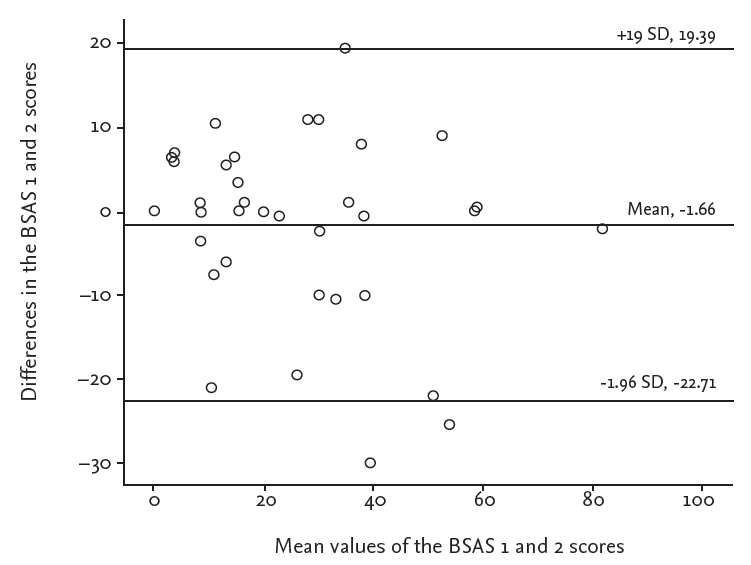
Bland-Altman plots of the mean difference between BSAS 1 and 2 scores (−1.66); most of the cases were between the upper and lower limits. BSAS, Behcet’s Syndrome Activity Scale.
The BSAS score was significantly correlated with the BDCAF score (r = 0.701, p < 0.001), and moderately correlated with the patient/physician VAS (r = 0.450, p = 0.011; r = 0.441, p = 0.001) at time 1, and with the BDQOL score at both times (r = 0.358, p = 0.001; r = 0.522, p = 0.001) (Table 3). The correlation of the patient/physician global assessment with the BDCAF and BDQOL was further analyzed by Spearman rank correlation. The BDCAF or BDQOL moderately correlated with the patient’s VAS (r = 0.403, r = 0.594), the physician’s VAS (r = 0.394, r = 0.462), and the BDCAF with BDQOL (r = 0.360, p < 0.005 for all).
DISCUSSION
Patient-reported outcome measures (PROMs), which are simple and applicable to most rheumatic diseases, are used in daily practice. Disease activity can be quantified using self-administered questionnaires. Treatment of rheumatoid arthritis (RA) patients that is guided by the use of quantitative measures is associated with better outcomes than the usual non-quantitative care [10]. In RA, PROMs are found to be as informative, if not more so, than physician-driven measures [11].
BD presents with heterogeneous organ involvement and has a fluctuating disease course [1]. There are currently no laboratory tests to inform of disease severity [1-3]. Quantitative measurements are useful in the assessment of BD, such as RA, but there are few direct measures of BD. The BDCAF was previously validated [4], but it is very time-demanding for clinicians to complete a BDCAF for every patient in a busy clinical setting. The BSAS is the first patient-reported assessment to measure BD activity [5]. In this study, a cross-cultural adaptation of the BSAS questionnaire was assessed in Korean patients with BD. Reliability and validity tests showed this to be a successful adaptation of the BSAS into the Korean language.
As a measure of reliability, there was good correlation for each item and the total scores of BSAS between times 1 and 2. The BDCAF and BDQOL were used as the gold standard to assess the validity of the BSAS. There was a high correlation between the BSAS and BDCAF, and a moderate correlation between the BSAS and the patient/physician VAS and the BDQOL. Forbees et al. [5] reported similar results. In a Turkish study [6], the BSAS correlated moderately with the BDCAF and the routine assessment of patient index data (RAPID) 3; the correlation between RAPID 3 and BDCAF was moderate. They also reported good correlation between the patient global assessment, BSAS, BDCAF, and RAPID 3 (r = 0.767, r = 0.583, and r = 0.686; p < 0.001 for all). In this study, the BDQOL was used for BD, and there were significant positive correlations between the total BDCAF scores and the patient/physician VAS or BDQOL, which represent reliability and reproducibility in our study.
This study was limited in that the patients were from a tertiary hospital, indicating that they likely did not represent a broader population of such patients. The main strength was that this study represents the first cross-cultural adaptation of the BSAS in South Korea, offering an accurate, easy-to-use tool to assess BD activity in Koreans.
In conclusion, the cross-cultural adaptation of the BSAS questionnaire was successful for use in Korea. The Korean version of the BSAS (BSAS-K) represents a reliable and valid instrument for measuring current BD activity. The BSAS-K can be used as a simple patient-derived measurement to assess clinical BD activity in a busy clinical setting.
KEY MESSAGE
1. Cross-cultural adaptation of the Korean version of Behcet’s Syndrome Activity Score (BSAS-K) was successfully carried out.
2. There was good agreement and correlation between the BSAS score and the Behcet’s Disease Current Activity Form/Behcet’s Disease Quality of Life/global assessment of disease activity by patients or physicians.
3. The BSAS-K is a useful instrument to measure Behcet’s disease activity as a measure of patient-reported outcomes.
Notes
No potential conflict of interest relevant to this article was reported.
References
Appendices
Appendix 1. Behcet’s syndrome activity scale (Korean version).