Clinical characteristics of spontaneous coronary artery dissection in young female patients with acute myocardial infarction in Korea
Article information
Abstract
Background/Aims
We aimed to evaluate the prevalence, characteristics, and clinical outcomes of spontaneous coronary artery dissection (SCAD) in young female patients with acute myocardial infarction (AMI).
Methods
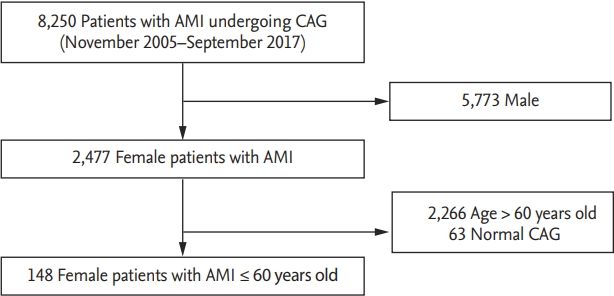
We identified 8,250 patients diagnosed with AMI who underwent coronary angiogram from the Chonnam National University Hospital database, Gwangju, Korea, between November 2005 and September 2017. A total of 148 female patients aged less than 60 years with a history of AMI were retrospectively studied and the characteristics and clinical outcomes were evaluated for all SCAD patients.
Results
Among female patients with AMI aged less than 60 years, the prevalence of SCAD was 8.78% (13 of 148). Based on the angiographic classification, type 2 SCAD was most commonly observed on angiograms in 69.2% of the cases (nine of 13), followed by type 3 in 23.1% (three of 13), and type 1 in 7.7% (one of 13). Furthermore, the left anterior descending (LAD) artery was the most commonly affected coronary artery (76.9%, 10 of 13 cases) and the distal segments of the coronary arteries were the most common sites of SCAD (92.3%, 12 of 13). Regarding the clinical outcomes, one of 13 patients experienced repeat revascularization during the following 31 months.
Conclusions
The prevalence of SCAD was 8.7%, indicating that SCAD is not rare, among female patients aged less than 60 years with AMI in Korea. Type 2 SCAD was most commonly observed on angiogram. Moreover, the distal portion of the LAD was the segment most commonly affected by SCAD. The long-term clinical outcomes were favorable in patients surviving SCAD.
INTRODUCTION
Although the true prevalence of spontaneous coronary artery dissection (SCAD) is still uncertain, SCAD has been considered to be an infrequent cause of acute coronary syndrome (ACS), including acute myocardial infarction (AMI), with a prevalence of approximately 0.16% to 2.0%, detected on a coronary angiogram (CAG) [1-5]. However, the use of optical coherence tomography, providing a high resolution of 10 μm, enables detection intramural hematoma and a false lumen for SCAD diagnosis, and it revealed a higher prevalence of 4.0% in ACS patients [6-9]. Furthermore, SCAD is a frequent cause of ACS in young female patients [2-4,10].
With respect to the clinical outcomes, mid- to longterm mortality rate is low in patients who survive SCAD [3,11-13]. In contrast, the overall rate of major adverse cardiac events (MACE), such as death, recurrent AMI and urgent revascularization, is significant and it has been reported in 14.6% to 47.4% of cases during a 3- to 10-year follow-up period [11-13].
However, the data on SCAD in patients with AMI, especially young females who rarely have traditional cardiovascular risk factors, are limited, although AMI is the leading cause of mortality in the Asia-Pacific population, including Korea [14,15]. Therefore, the aim of this retrospective observational study was to investigate the prevalence, characteristics, and clinical outcomes of SCAD in young female patients with AMI in Korea.
METHODS
Study population
The study population was derived from the Chonnam National University Hospital database, Gwangju, Korea, between November 2005 and September 2017. Female patients aged less than 60 years with AMI were divided into two groups, namely, the SCAD and non-SCAD groups. We compared the baseline clinical characteristics, laboratory findings, and medication between the groups. Moreover, the clinical characteristics and outcomes were evaluated in all SCAD patients. The study protocol was approved by the Institutional Review Board of the Chonnam National University Hospital (approval number: CNUH 05-49) and the need for informed consent was waived.
Angiographic classification of spontaneous coronary artery dissection
SCAD was diagnosed and classified according to the criteria suggested by Saw as shown in Fig. 1 [16]. All CAGs were assessed for the diagnosis of SCAD by two independent interventional cardiologists (Y.K. and X.H.), who were blinded to the history of the patients. Type 1 refers to the classic appearance of arterial wall contrast staining or multiple radiolucent lumens (Fig. 1A). Type 2 refers to an abrupt caliber reduction from the normal diameter to diffuse smooth narrowing with or without the restoration of distal vessel caliber (type 2a and 2b SCAD, respectively) (Fig. 1B and 1C). Type 3 SCAD refers to tubular stenosis that mimics atherosclerosis requiring intracoronary imaging to confirm diagnosis (Fig. 1D). Regarding coronary artery segment classification, two interventional cardiologists determined each coronary artery segment according to the American Heart Association classification [17].
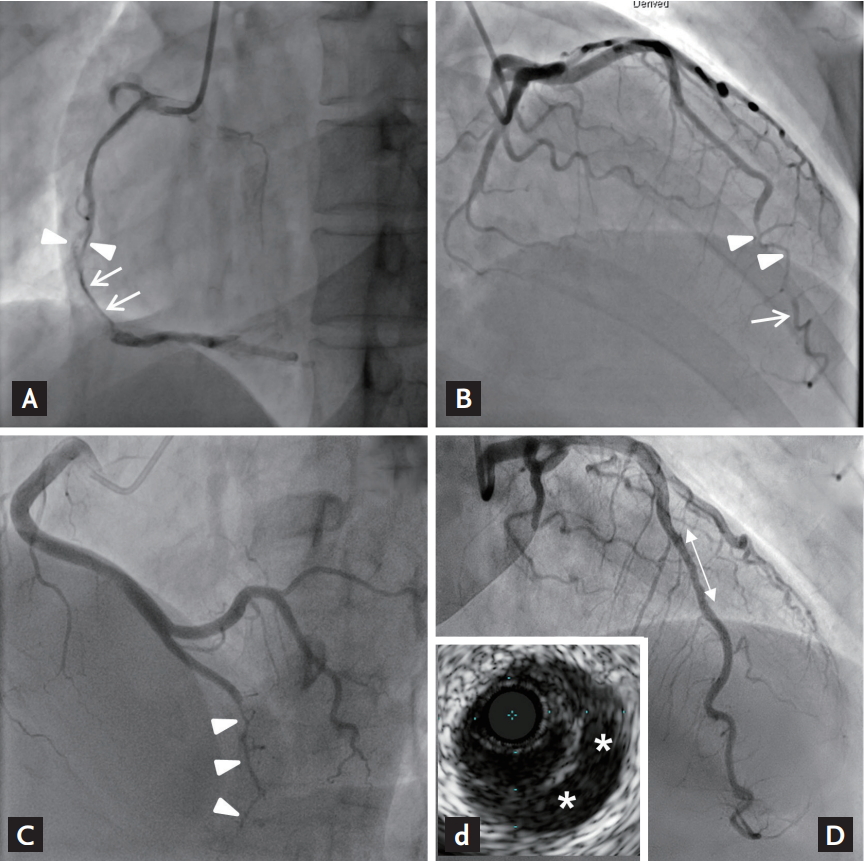
Angiographic classification of spontaneous coronary artery dissection. (A) Patient no. 9: type I, arterial wall contrast staining (arrows) or multiple radiolucent lumens (arrowheads). (B) Patient no. 7: type 2a, abrupt caliber reduction (arrowheads) with restoration of distal vessel caliber (arrow). (C) Patient no. 13: type 2b, abrupt caliber reduction without restoration of distal vessel caliber (arrowheads). (D) Patient no. 5: type 3, tubular stenosis mimicking atherosclerosis (double-point arrow), and confirming false lumen (asterisks) by intravascular ultrasound (d).
Statistical analysis
All continuous variables were expressed as mean with standard deviation (SD) or median with interquartile ranges (IQR), as appropriate. All categorical variables were reported as numbers with percentages. The continuous variables were compared using the unpaired t test or Mann-Whitney U test, as appropriate. The categorical variables were analyzed by chi-square test or Fisher’s exact test. All analyses were two-tailed, and a p < 0.05 was considered significant. All statistical analyses were performed using SPSS for Windows software version 21.0 (IBM Co., Armonk, NY, USA).
RESULTS
Between November 2005 and September 2017, we identified 8,250 patients diagnosed with AMI who underwent CAG. Male (n = 5,773) and female patients aged more than 60 years (n = 2,266) were excluded. Furthermore, patients with normal coronary artery (n = 63), defined as less than 30% stenosis on CAG by quantitative coronary angiography, were also excluded. Therefore, we selected a total of 148 female AMI patients aged less than 60 years (Fig. 2).
Prevalence of SCAD in the study population
From among 148 female patients with AMI aged less than 60 years, 13 patients presenting with SCAD were identified. Therefore, the overall prevalence of SCAD in this study population was 8.78% (13/148) (Fig. 3).
Baseline characteristics and laboratory findings
Baseline characteristics and laboratory findings are shown in Table 1. Mean age was similar for the SCAD and non-SCAD group (52.1 ± 5.8 vs. 53.7 ± 5.7, p = 0.350). There were no pregnant patients in either of the groups. With respect to the baseline characteristics, there was no significant difference between the two groups. As far as laboratory findings are concerned, lipid profiles were significantly different in the two groups. The salient difference here was that the high density lipoprotein cholesterol level was significantly higher in the SCAD group (p < 0.001). Furthermore, the peak troponin-I level was significantly lower in the SCAD group than in the non-SCAD group (5.1 ng/dL vs. 17.1 ng/dL, p < 0.001).
Coronary angiographic findings and medication at discharge
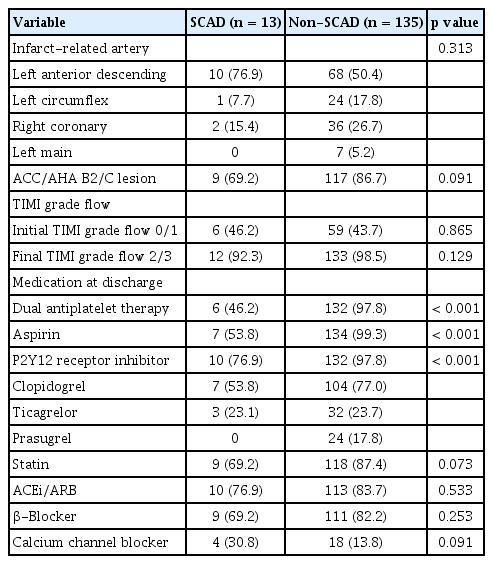
Coronary angiographic findings and medication at discharge are shown in Table 2. With respect to the infarct-related artery, there was no significant difference between the two groups. However, SCAD was not observed in the left main coronary artery. Lesion severity and Thrombolysis In Myocardial Infarction (TIMI) grade flow were similar between the two groups. Regarding discharge medication, antiplatelet agents, including aspirin and P2Y12 receptor inhibitor as the dual antiplatelet therapy (DAPT) (46.2% vs. 97.8%, p < 0.001), aspirin (53.9% vs. 99.3%, p < 0.001), and P2Y12 receptor inhibitor (76.9% vs. 97.8%, p < 0.001), were less frequently prescribed to the SCAD group compared to the non- SCAD group.
Characteristics of patients with SCAD
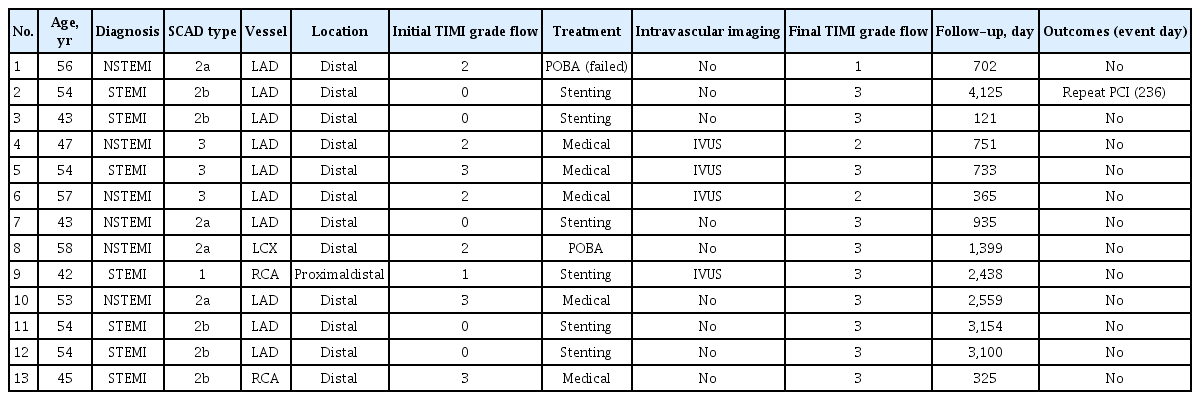
Characteristics of patients with SCAD are shown in Table 3, Fig. 3. Regarding angiographic classification of SCAD, type 1 was observed in one (7.7%), type 2 was observed in nine (69.2%) (type 2a in four [30.8%] and type 2b in five [38.5%]), and type 3 was observed in three patients (23.1%) (Fig. 3). Among 13 patients with SCAD, the coronary artery involved was the left anterior descending (LAD) artery, the right coronary artery, and the left circumflex artery in 10 (76.9%), two (15.4%), and one (7.7%), respectively. Twelve SCAD lesions (93.3%) were observed only in the distal portions of the coronary arteries. TIMI grade flow was 0/1 in six (40%), and all these patients were treated with percutaneous coronary intervention (PCI) with stent implantation. Intravascular ultrasound guidance was performed in four (30.8%). The final TIMI grade flow of 2/3 was achieved in most patients (92.3%). During a median follow-up of 935 days (range, 121 to 4,125), one patient required repeat PCI (Table 3).
DISCUSSION
In this study of female patients under the age of 60 years with AMI, we found that the prevalence of SCAD was 8.78%. Using angiographic classification, the most common SCAD angiographic appearance was type 2 in 69.2% of cases (nine of 13), followed by type 3 in 23.1% (three of 13), and type 1 in 7.7% (one of 13). Furthermore, SCAD was frequently observed in the LAD (76.9%, 10 of 13) and the distal segments of the coronary arteries were the most common sites of SCAD (92.3%, 12 of 13). Regarding the clinical outcomes, one of 13 patients experienced repeat revascularization during the following 31 months. To our knowledge, this is the first Korea study reporting the prevalence and characteristics of SCAD patients presenting with AMI.
Several studies have assessed the prevalence of SCAD in young female patients with ACS including ST-elevation myocardial infarction (STEMI). Vanzetto et al. [2] reported that the prevalence of SCAD was 8.7% (12 of 138) in female ACS patients under 50 years of age and reached 10.8% (eight of 74) in cases of STEMI. However, the prevalence was reported as 24.2% (16 of 66) among young women with AMI (aged ≤ 50) in a retrospective single-center study [10]. Another retrospective single-center study reported an identical prevalence of 24.2% (16 of 66) in female patients under the age of 60 years with AMI [4]. Moreover, the Japanese multicenter registry described a prevalence of SCAD of 34.6% (45 of 130) in female patients aged under 50 with AMI [3]. Although it is difficult to explain the discrepancy in the prevalence of SCAD in these different studies, the present study supported the view that SCAD is not rare among young female AMI patients undergoing CAG. Therefore, interventional cardiologists must consider SCAD in the differential diagnosis of the etiology of AMI in young women.
Several studies have reported that the most common angiographic appearance is type 2 in 52% to 67% of all SCAD cases, which is comparable to the results in the present study [3,18,19]. With respect to the affected artery and segment, the LAD and mid to distal segments are each commonly affected [18]. The present study also showed similar results in that the involvement of the LAD and distal segments is common, albeit in a relatively higher proportion than in other studies. Therefore, interventional cardiologists should look carefully at the distal segment of the LAD when performing CAG in young female patients presenting with AMI.
Expert opinion supports the view that overall conservative treatment is preferable in patients with SCAD as spontaneous healing of the affected artery was observed in most cases with medical treatment and PCI was associated high failure rates [16,20,21]. However, Buccheri et al. [22] reported that physicians preferred invasive treatment in about 40% of SCAD patients presenting with ACS, although most of the SCAD patients were treated conservatively in the literature surveyed. In our study, the patients with TIMI grade flow 0/1 were treated with PCI with stent implantation. Among patients with TIMI grade flow 2/3, two patients were treated with PCI with plain old balloon angioplasty, but PCI failure (TIMI grade flow 2 to 1) was observed in one case (patient no. 1). In contrast, the present study showed good long-term clinical outcomes in patients with TIMI grade flow 2/3 who treated with medical treatment. Therefore, conservative treatment may be a good management option for SCAD patients presenting with AMI, unless there is flow limitation.
There is a scarcity of data regarding the role of DAPT in SCAD patients. Patients with SCAD who undergo stenting should receive DAPT according to current revascularization guidelines [23]. In the present study, DAPT was prescribed in all SCAD patients who were treated with stent implantation (six of six). With respect to SCAD patients with conservative management, a further prospective study should be needed to confirm the benefit of antiplatelet therapy.
With regard to the clinical outcomes, several studies have reported that long-term mortality is low, approximately 0% to 8.0% during a follow-up of 2.8 to 10 years [3,11-13,18]. This study showed that the prognosis was favorable and the frequency of cardiovascular events was low with only one patient (7.7%) requiring repeat PCI due to a stent fracture. In this patient, subsequent follow-up of 10.8 years revealed no recurrence of cardiovascular events. Except for one adverse event, there were no MACE including death during the follow-up period.
There were several limitations in the present study. Firstly, this study was a single-center, retrospective study with the inherent limitations of a small sample size. Therefore, selection bias was virtually unavoidable. Secondly, in relation to patients with type 3 SCAD, we included only those who were confirmed by an intravascular imaging tool. This might have led to underestimation of the prevalence of type 3 SCAD as patients in this category who did not undergo intravascular imaging might have been inadvertently excluded. Furthermore, there was no evaluation of lesion characteristics with intravascular imaging to confirm a diagnosis in patients with type 2 SCAD. Therefore, the prevalence of type 2 SCAD might have been overestimated in this study. Thirdly, we did not examine the possibility of fibromuscular dysplasia, the most frequent predisposing arteriopathy, in the SCAD group. Despite these limitations, this study is expected to prove helpful to physicians in understanding the characteristics of patients with SCAD in Korea.
In conclusion, prevalence of SCAD was 8.78% in female patients with AMI under the age of 60 years. Moreover, type 2 and involvement of the distal portion of the LAD were the most common angiographic appearance and segment of SCAD, respectively. Long-term clinical outcomes were favorable in patients surviving SCAD. Therefore, increased awareness of SCAD is needed when CAG is performed in young female patients presenting with AMI as SCAD is not rare in these patients.
KEY MESSAGE
1. Between 2005 and 2017, the prevalence of spontaneous coronary artery dissection (SCAD) was 8.78% among female patients aged less than 60 years with acute myocardial infarction from single-center data in Korea.
2. Type 2 SCAD was most commonly observed on angiograms, and the distal portion of the left anterior descending artery was the most commonly affected segment in the patients.
3. Long-term clinical outcomes were favorable in patients surviving SCAD.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported by a grant from Chonnam National University Hospital Biomedical Research Institute (BCRI8015) and by a grant from the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI13C1527).