The effect of Hericium erinaceum on the prevention of chemically induced experimental colitis in rats
Article information
Abstract
Background/Aims
The aim of this study is to investigate the effects of the Hericium erinaceum on an experimental colitis model.
Methods
Twenty-four Wistar albino were included in this study. Rats were divided into three groups. Group 1 (n = 8) was sham group. Group 2 is the group of chemically induced by intrarectal administration of trinitrobenzene sulfonic acid (TNBS) resulting in colitis. Group 3 (n = 8) is the group that was treated 7 days before and 7 days after with H. erinaceum resulting in colitis. The activity of colitis was evaluated macroscopically and microscopically in rats. In other words, nitric oxide (NO) levels, malondialdehyde (MDA), interleukin 6 (IL-6), nuclear factor-kappa B (NF-κB) and, tumor necrosis factor-α (TNF-α) in addition to the myeloperoxidasem (MPO) activities was determined.
Results
The rate of TNBS-induced colitis caused to increase the level of MDA activities meaningfully in the colitis group than the control group. The results indicated that MDA (p = 0.001), NO (p = 0.001), IL-6 (p = 0.001), MPO (p = 0.878), TNF-α (p = 0.001), and NF-κB levels of treatment group decreased in the blood and colon tissues because of the H. erinaceum treatment when compared to the colitis group. H. erinaceum treatment was related to the declining of MDA, NF-κB, NO, IL-6, and TNF-α levels.
Conclusions
H. erinaceum had a positive effect on the colitis by reducing oxidative damage in blood and tissue.
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic inflammatory disease of unknown cause, and effective treatment options have not been discovered [1]. Recent data increasingly indicate that signaling molecules and pathways are responsible for the onset and progression of the disease. There is evidence that the increased regulation of inflammatory mediators, such as cytokines, chemokines, and adhesion molecules, plays an essential role in both human and experimental colitis models [2,3]. Nuclear factor-kappa B (NF-κB) is responsible for the gene expression regulation of inflammatory mediators such as tumor necrosis factor-α (TNF-α), interleukin 6 (IL-6), and nitric oxide synthase (iNOS) [4,5]. NF-κB is a key molecule involved in intestinal inflammation [6]. Studies that investigate the effect of anti-inflammatory drugs on the insula in IBD are difficult. For this reason, studies use animal models to investigate approaches to reduce inflammation and to activate various pathways.
Hericium erinaceus (HE), known as lion’s mane, is a medicinal mushroom and has been historical used in traditional Chinese medicine. HE mycelium and fruit bodies show great promise as substances that improve health such as brain and nerve health. The animal and in vitro experiments that investigated substances extracted from HE showed good results. In particular, the mycelium extract has been used clinically as an anti-inflammatory agent and in cytotoxic and cardiovascular antineoplastic research as an antibacterial and neuroprotective agent [7,8]. HE represents an important opportunity to treat Alzheimer’s and Parkinson’s diseases because HE is used as an anti-inflammatory medicine and promotes nerve growth factor gene expression.
The aim of this study was to evaluate the protective effect of HE treatment in a TNBS-induced colitis rat model.
METHODS
Twenty-four Wistar albino rats (225 to 275 g) were included in this study. Rats were kept in wire-mesh bottomed cages under a 12-hour light/12-hour dark cycle at a constant room temperature (22ºC ± 2ºC). A standard chow diet and water were provided. The study was approved by the Istanbul University ethics committee (number 2013-88).
Chemical reagent
2,4,6-Trinitrobenzene sulfonic acid (TNBS) was purchased from Sigma-Aldrich. The chemical formula of TNBS is C6H3N3O9S. Its other names are picrylsulfonic acid and trinitrobenzene. It has extreme oxidative properties. HE plays an antioxidative role because of its active substances and reducing ability. Its ethanol and hot water extracts have very high antioxidant activity in vitro. HE was purchased from Health for Life Trading Co., Ltd. HE (250 and 500 mg/kg/day body weight) was administered orally to rats.
Induction of colitis
Rats were slightly anesthetized with ether after a 24-hour fast. A 5-French polyurethane cannula was placed into the anus, and the tip was inserted to 8 cm proximal to the anal verge [9]. TNBS (10 mg/0.25 mL), which was dissolved in 50% ethanol, was inoculated into the colon through the cannula to induce colitis. Then, the rats were headstand to observe complications. The rats with complications were excluded from this study, and a new experimental subject was included in this study.
Three groups with an equal number of animals were formed. Group 1 was the sham group (n = 8). After catheterization, the anesthetized rats were substituted with 0.9 mL of 0.8% NaCl through the rectum. Group 2 was the colitis group (n = 8). To induce colitis, after catheterization through rectum, the mixture (35 mg/0.2 mL TNBS and 0.4 mL 50% ethanol) was administered to the anesthetized rats. Group 3 was the treatment group (n = 8). After catheterization and colitis induction, 35 mg/0.2 mL and 0.4 mL 50% of ethanol were administered to the anesthetized rats rectally administered TNBS. Additionally, the rats received HE (250 and 500 mg/kg/day body weight) orally 7 days before colitis induction and 7 days after colitis induction. The colon weight and colon length of each rat were recorded.
Surgical procedure
The surgical procedure under sterile conditions was applied to the experimental group on the 7th day of treatment. Intramuscular administration of 70 mg/kg ketamine hydrochloride and 7 mg/kg xylazine was used to anesthetize all Wistar albino rats. General anesthesia and spontaneous respiration were used. Povidone iodine was used to clean the abdominal region. The part of the colon with induced colitis was transected distal to the rectum as low as possible, and a nearly 9 cm long colonic segment was collected. The segment was opened longitudinally, and 0.8% saline solution was used to rinse the feces. For the analysis of biochemical parameters, 4 mL blood was collected. For biochemical and histopathological research, tissue samples were collected and transferred to a 0.8% NaCl solution or 9% formaldehyde solution. Blood samples were centrifuged and stored at –80ºC for biochemical research. Last, rats were sacrificed using a lethal dose of thiopental sodium.
Histopathological evaluation
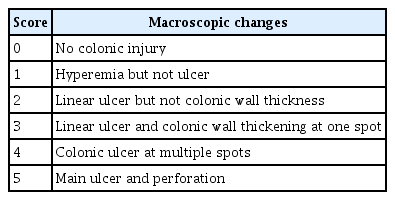
Macroscopic score
Macroscopic changes in the colonic mucosa were categorized into five levels (Table 1).
Microscopic score
The colon was divided into two parts for pathologic and biochemical studies after the macroscopic evaluation of the mucosa. The histopathological study was performed at Istanbul University. Colon tissue was separated into 5 mm sections and fixed in 9% formaldehyde [10]. After embedding in paraffin and staining with hematoxylin-eosin, 5 mm sections were prepared and evaluated with a light microscope. Inflammatory infiltration, thrombosis, apoptosis, necrosis, and vascular congestion were analyzed. All tests and microscopic alterations in the mucosa were evaluated from 0 to 3 by a histopathologist blinded to the groups.
Biochemical evaluation of the tissue and blood
For the biochemical assays, blood and terminal ileum were obtained. Blood samples were collected using a sterile needle by intracardiac puncture. Next, blood samples were obtained, centrifuged and separated into serum samples. Nitric oxide (NO), NF-κB, TNF-a, malondialdehyde (MDA), myeloperoxidasem (MPO), and IL-6 were evaluated. Tissues were rinsed with serum, weighed and homogenized for biochemical investigation. Distal colon samples were collected for biochemical evaluation. To detect inflammation, IL-6, MPO and TNF-a were evaluated. To determine the rate of apoptosis, NO and MDA, which can indicate the extent of oxidative damage, were investigated.
MPO measurement
Tissues were homogenized, incubated in 0.5% hexadecyl-trimethylammonium bromide (pH 5.5) and 0.026% ortho-dianisidine dihydrochloride, and 0.018% H2O2 was added. The reaction time was half an hour. The reaction was validated with sodium azide. All tests were performed twice and checked.
MDA measurement
MDA and thiobarbituric acid were examined by using a spectrophotometric procedure. Then, 0.2 mL of serum, 25 µL of butylated hydroxytoluene (pH 7.4) and 0.8 mL of tamponated phosphate were mixed. The prepared solution was mixed with 0.5 mL of 30% trichloroacetic hydro barbituric acid, put on ice for 2 hours and then centrifuged at 25°C for 15 minutes at 2,000 ×g. After centrifugation, ethylene diamine, tetra acetic thiobarbituric acid (0.070 mM) and 0.9% thiobarbituric acid (0.20 mL of 0.1 mol/L) was added to each mL. The supernatant was reserved in boiled water and left to cool at room temperature for 15 minutes. Spectrometric measurement was performed for the last prepared supernatant at 532 nm wavelength. The outcomes were recorded as nmol/mL.
NO measurement
The serum NO level was analyzed with Griess reagent. For the first phase, nitrate reductase was used to degrade the nitrate in the serum. For the second phase, nitrogen purple was improved by Griess reagent. Zinc sulfate was added to this mixture and centrifuged at 10,000 ×g for 4 minutes. The measurement was performed using azo dye by chromatographic spectrometer. The outcomes were recorded as nmol/mL.
TNF-α and IL-6 measurements
An enzyme-linked immunosorbent assay was used to measure TNF-a and IL-6. The lowest values that could be determined were 0.12 pg/mL and 0.03 pg/mL for TNF-a and IL-6, respectively.
NF-κB measurement
The colon of rats was embedded in 10% buffered formalin. Paraffin blocks were cut into 5 µm slices. The paraffin-embedded specimens were deparaffinized and postfixed in 100% acetone for 5 minutes to block endogenous peroxidase activity. Antigen retrieval was performed, and the sections were incubated with primary rabbit polyclonal anti-NF-κB antibody (anti-rabbit P50Ab-2) for 30 minutes at room temperature. Then, the slides were treated with biotinylated secondary antibody and streptavidin-conjugated horseradish peroxidase. Hematoxylin was used to stain for visualization [11].
Statistical evaluation
The results were evaluated as the mean and standard deviation. All data are expressed as the mean ± SD. Kruskal-Wallis and analysis of variance were used for statistical analysis, and p < 0.05 was accepted as significant (SPSS version 25.0 for Windows, IBM Co., Armonk, NY, USA).
RESULT
Macroscopic colitis score
The macroscopic score was markedly increased in all rats administered TNBS compared with the rats in the sham group (p < 0.001). Macroscopic injury of the colon on the 7th day after TNBS administration indicated hyperemia, ulceration, colonic wall thickness and severe adhesions between the colon and other organs. HE treatment markedly reduced the macroscopic score in TNBS-treated animals (p < 0.001).
To investigate colitis formation, the length of the colon and the weight of the colon in the sham, HE treatment and nontreatment groups were compared. In the HE-treated group, the length of the colon was similar to that of the sham group, and the weight of the colon was similar to that of the sham group. Colon weight was increased, and colon length was decreased in the untreated group (Fig. 1).
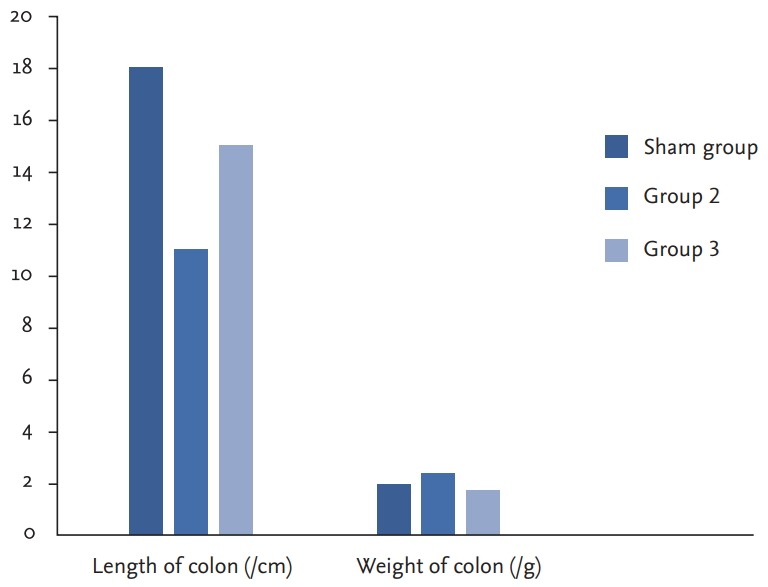
Graph of colon length and weight for sham group, group 2, and group 3. The sham group, the treatment and nontreatment groups were compared to evaluate colitis formation. In the group 3, the colon length and the weight was close to sham group whereas, the length of colon was decreased and the weight of colon increased for the group 2.
Microscopic colitis score
The microscopic score was markedly higher in all rats administered TNBS than in the rats in the sham group (p < 0.001) (Fig. 2). After treatment with HE and TNBS, the pathologic score decreased markedly (p < 0.001). Fig. 3 shows that HE administration resulted in less mucosal injury, healed mucosal structure, and epithelial integrity. According to immunohistochemistry analysis, NF-κB expression was higher in the rats from group 2 higher than in rats from group 3.
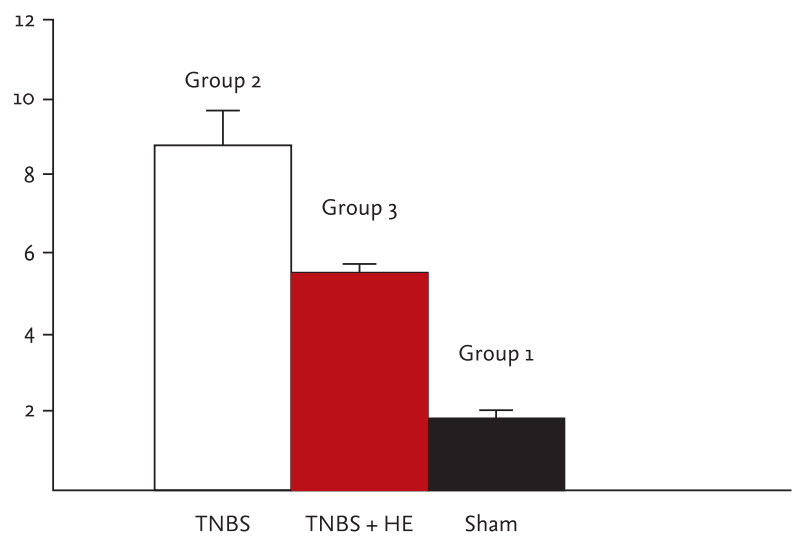
Microscopic colitis score. The significance between 1 and 2 and 3 is p < 0.05; 3 and 2 is p < 0.05. TNBS, trinitrobenzene sulfonic acid; HE, Hericium erinaceus.
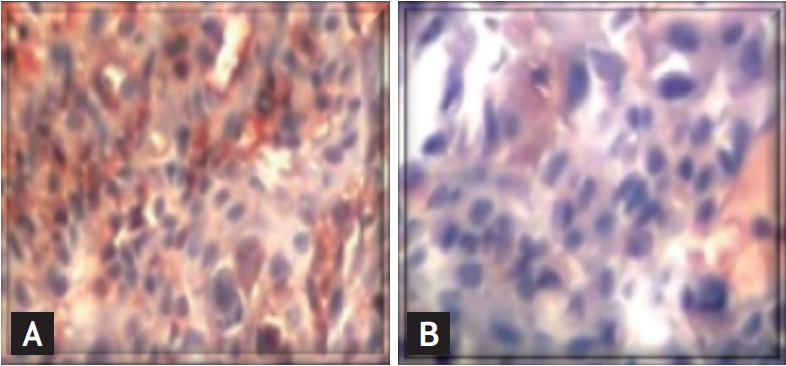
Comparison of the inflammation between group 2 and group 3 (H&E stain, ×400). (A) Up-regulation of the nuclear factor-kappa B (NF-κB) expression in the colon in the trinitrobenzene sulfonic acid-induced colitis. (B) Colon from the Hericium erinacues treated rats. There was a minimum NF-κB expression.
Biochemical results
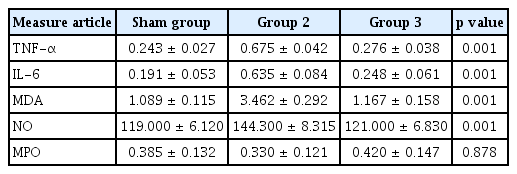
The colonic MDA levels are shown in Fig. 4. The oxidative damage parameters (NO and MDA levels) were compared among the groups. The level of the sham group was markedly different from that of the treatment group and colitis group. The blood and tissue levels of NO and MDA were markedly decreased in the HE-treated group compared with the colitis group (blood NO, p = 0.001; tissue NO, p = 0.001; blood MDA, p = 0.001; tissue MDA, p = 0.001). The comparison of oxidative damage is shown in Table 2.
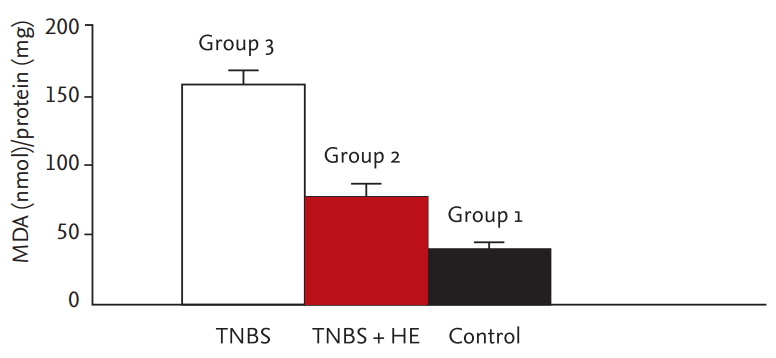
Colonic malondialdehyde (MDA) levels of all groups. Values with different letters have significance according to analysis of variance test. The significance between 2 and 3 is p < 0.001. TNBS, trinitrobenzene sulfonic acid; HE, Hericium erinaceus.
IL-6 and TNF-α levels in blood and tissue were analyzed as inflammatory parameters. The levels in the treatment group and colitis group results were markedly different from the levels in group 1 (p = 0.001). Compared with the colitis group, group 3 showed substantially decreased levels. Another parameter of inflammation in tissue is MPO level. The MPO levels of the treatment and colitis groups were not significantly different (p = 0.878). The inflammatory damage results are shown in Table 2.
The level of NF-κB expression in the untreated group was high (p < 0.01). The NF-κB expression level was lower in the HE treatment group than in the TNBS-induced colitis model (Fig. 3).
DISCUSSION
HE is an edible and medicinal mushroom and has also been used as a traditional Chinese medicine. Various studies have shown that the content of the HE is useful for medical purposes because of its high protein, low fat and high nutritional value [12,13]. HE contains erinaceus hericenone, CH [14,15], erinacines AK [16,17], orcinol derivatives (mycelium) [18], sialic acid-binding lectin [19], ergosterol and beta-sitosterol [20]. Since hericenone and erinacine strongly stimulate nerve growth factor synthesis, which allows for neuron repair and regeneration, HE increases nerve growth factor synthesis, and its compound activity slows neuronal cell death [17]. For this reason, HE can be used as a nutritional therapy in the treatment of dementia, Alzheimer’s disease and Parkinson’s disease. The orcinol derivatives obtained from the mycelium of HE show antibacterial and antifungal activity against certain pathogenic microorganisms (such as Bacillus subtilis and Saccharomyces cerevisiae) [21]. Another component of HE, sialic acid-binding lectin, can be used to identify, quantify, localize, purify, and characterize many biomolecules (such as glycoconjugates). Sialic acid plays an important role at the protein and cell levels in the biological recognition mechanisms, and lectins can be used as specific probes for sialic acid species, which act as molecular markers in pathological and physiological development [22]. Ergosterol is a major fungal sterol, and beta-sitosterol reduces the absorption of cholesterol in the intestinal lumen. In the study by Mbambo et al. [23], these stressors were shown to have antifungal activity. HE also has a prebiotic effect; it contained the polysaccharide β-glucan and the monosaccharides mannose (approximately 55.70%), arabinose (approximately 0.7%), rhamnose (approximately 3.75%), xylose (approximately 1.60%), galactose (approximately 5.12%), and glucose (approximately 33.10%) [24]. Since HE contains physiologically important compounds, there are some studies on gastritis and gastrointestinal infections. Extracts of HE mycelium have been indicated to inhibit gastric mucosal damage and improve gastric ulcers [25], with the inclusion of HE in the diet improved colonic health and IBD, and HE has anti-inflammatory activity [26,27].
The results of our study suggest that treatment with HE has a positive impact on colitis in TNBS-induced rats by reducing oxidative damage in blood and tissue. This is indicated by the significantly decreased NF-κB, MDA, NO, IL6, and TNF-α levels in blood and tissue. Additionally, the current study shows macroscopic and microscopic colitis scores that indicate less mucosal damage and improved mucosal structure.
IBD is a chronic, recurrent disease characterized by idiopathic inflammation of the gastrointestinal tract whose pathogenesis is not known precisely. IBD mainly consists of two clinical forms, Crohn’s disease (CD) and ulcerative colitis (UC). UC is characterized by recurrent weakness of the mucosal layer of the colon [28]. It is most commonly seen in the rectum, spreading to other parts of the colon with continuity. CD is a disease of the digestive tract that may be anywhere from oral to annular, with focal, asymmetric, or transmural involvement [29]. Some clinical studies have shown that genetic factors increase the risk of developing IBD [30]. The effects of environmental factors on the formation of IBD and processes are not fully understood. Clinical and epidemiological studies indicate that this disease can develop in genetically susceptible individuals after exposure to different antigens or environmental factors. Exacerbations and healing may be associated with these factors. According to our results, HE can be a protective agent for IBD.
Immune factors play an effective role in the pathogenesis of IBD. Some cytokines are proinflammatory (IL-1, IL-2, IL-6, and TNF-α), and some are anti-inflammatory (IL-8, IL-10, IL-11, and TNF-γ). HE treatment improved the inflammatory response of rats because treatment of TNBS-induced rats with HE resulted in increased levels of some anti-inflammatory cytokines compared to the untreated group [24]. Our study also showed that feeding TNBS-treated rats HE had a good inflammatory effect because the TNF-α and IL-6 levels of HE-fed rats were lower than those of the control group. It was also demonstrated that the administration of HE decreased inflammation, MDA levels and MPO activity in rats with TNBS-induced colitis.
Several compounds that initiate the immune response in the digestive tract have been identified, and experimental models, in which these compounds are generated, have been used to understand the pathogenesis of the disease and to develop drug treatments. TNBS is one of these compounds, and the disease model created by this substance is widely used in experimental studies since it produces effects similar to human UC [31,32]. In this study, cytokines such as TNF-α, MPO, NO, MDA, and IL-6 were investigated to determine the cellular activities of the TNBS-induced colitis model because cytokines are key signaling molecules of the intestinal immune system that play an important role in IBD. In vivo and in vitro studies indicate that the levels of proinflammatory cytokines such as TNF-α and IL-6 are elevated in IBD and that the severity of inflammation is directly proportional to the levels of these cytokines [33]. Moreover, the macroscopic colitis score of this study showed that colonic wall thickness, ulcers, severe adhesions between the colon and other organ hyperemia were observed on the 7th day in TNBS-induced rats. The comparison of the microscopic colitis scores of the TNBS-induced group and HE treatment group showed that the HE treatment group had less mucosal injury, improved mucosal structure, and epithelial integrity. In one study, the HE-treated group showed more pronounced improvement than did the TNBS-induced group. Mucosal erosion and ulcers were observed in the TNBS-induced group, and less injury was observed in the HE group than in the TNBS group. Our results clearly demonstrated that the HE extracts could promote the protection of the gastric mucosa and give positive results in the experimental models of IBD.
One previous study showed that HE extracts contributed to the growth of beneficial gut bacteria and ameliorated host immunity in an experimental IBD model. When TNBS-induced rats were treated with HE extracts, the expression levels of inflammatory factors were changed. MPO levels declined, and TNF-α levels in the peripheral blood and colon of the normal group were lower than those in TNBS-treated rats. In our study, according to the biochemical results, there was a decrease in MDA, NO, IL6, and TNF-α levels in the HE treatment group compared with the TNBS-induced colitis group. Treatment with HE enhanced all the inflammatory responses that we investigated.
Excess TNF-α expression damages epithelial barriers and induces apoptosis in epithelial cells [34]. In accordance with the literature, TNF-a expression was more intense in the areas of inflammation and ulceration in the colon mucosa of the colitis group. Zhou et al. [35] showed that TNF-a expression was increased in a TNBS-induced colitis model. This study showed that HE treatment decreased TNF-α and IL-6 levels because the TNF-α and IL-6 levels in the TNBS-induced colitis model were significantly higher than those in the HE group.
One of the previous observations showed that MPO levels in the HE group were lower than those in the colitis group, and HE extracts decreased colon inflammation in rats with IBD [22]. When MPO activity was examined, the values obtained from the colitis group were considerably higher than those of the control group (p < 0,05), and inflammation was observed. The MPO levels measured at the tissue level were significantly lower in the treatment groups than those in the colitis group (p < 0.05). Furthermore, the MDA level of the colonic tissues was found to be increased in the TNBS-treated group compared to the sham group. Treatment with HE caused a significant decrease in the MDA level in TNBS-induced colitis rats.
NF-κB proteins are transcription factors that control apoptosis, cell cycle progression, cell growth, and differentiation. The NF-κB pathway is involved in colonic inflammation, and increased NF-κB protein expression has been observed in the mucosa of patients with unspecific colitis, UC, and CD [36]. Proinflammatory cytokines induce NF-κB activation in different cell types, and this could be initiated by oxidative stress, which has a significant role in the pathogenesis and progression of IBD. Our biochemical findings also indicate that NF-κB expression decreased with HE administration, whereas NF-κB overexpression was observed in TNBS-induced colitis. The inhibition of NF-κB activation could be a possible treatment strategy for IBD.
As a limitation of this study, we analyzed MDA, NO, TNF-α, MPO, and NF-κB values to measure oxidative damage, which is similar to some studies [5,35,37]. The antioxidant effects of HE, the total antioxidant concentration and the protein expression levels of heme oxygenase-1 (HO-1) and NAD(P)H:quinone oxidoreductase (NQO1). were not analyzed in this study so that there was not much data complexity during the experimental phase and to ensure that the costs of the experiment did not increase. In future studies by our group, these values will be examined.
In conclusion, oral HE treatment showed good efficacy in the experimental colitis model. Biochemical indexes and microscopic and macroscopic colitis scores were analyzed. TNF-α, MPO, NO, MDA, and IL6 cytokines, which affect TBNS-induced colitis models, were examined, and TNF-α, MPO, NO, MDA, and IL6 levels were lower in the HE treatment group than in the colitis groups. Less mucosal injury was detected in the HE treatment group than in the colitis group. In light of these results, HE has clinical potential for the improvement of IBD because HE has an anti-inflammatory properties. The main disadvantage of oral therapy with HE is the unknown dose required for the anti-inflammation effect. For this reason, this issue will be investigated in future studies.
KEY MESSAGE
1. Hericium erinaceum had a positive effect on the colitis by reducing oxidative damage in blood and tissue.
2. Hericium erinaceum can be used as a nutritional treatment for inf lammatory bowel disease patients
3. The long-term effect used of Hericium erinaceum should be studied in more detailed.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
I would like thank Dr. Serap Durmus for help with the manuscript, data correction and biochemical results.