Efficacy and safety of first-line afatinib in older patients with advanced EGFR-mutated non-small cell lung cancer
-
Mi-Hyun Kim1,2,3, Hayoung Seong2,3, Soo Han Kim2,3, Min Ki Lee1,2, Insu Kim4, Kyung Soo Hong5,6, June Hong Ahn5,6, Jung Seop Eom1,2,3

- Received August 1, 2024; Revised October 8, 2024; Accepted November 4, 2024;
- Abstract
-
- Background/Aims
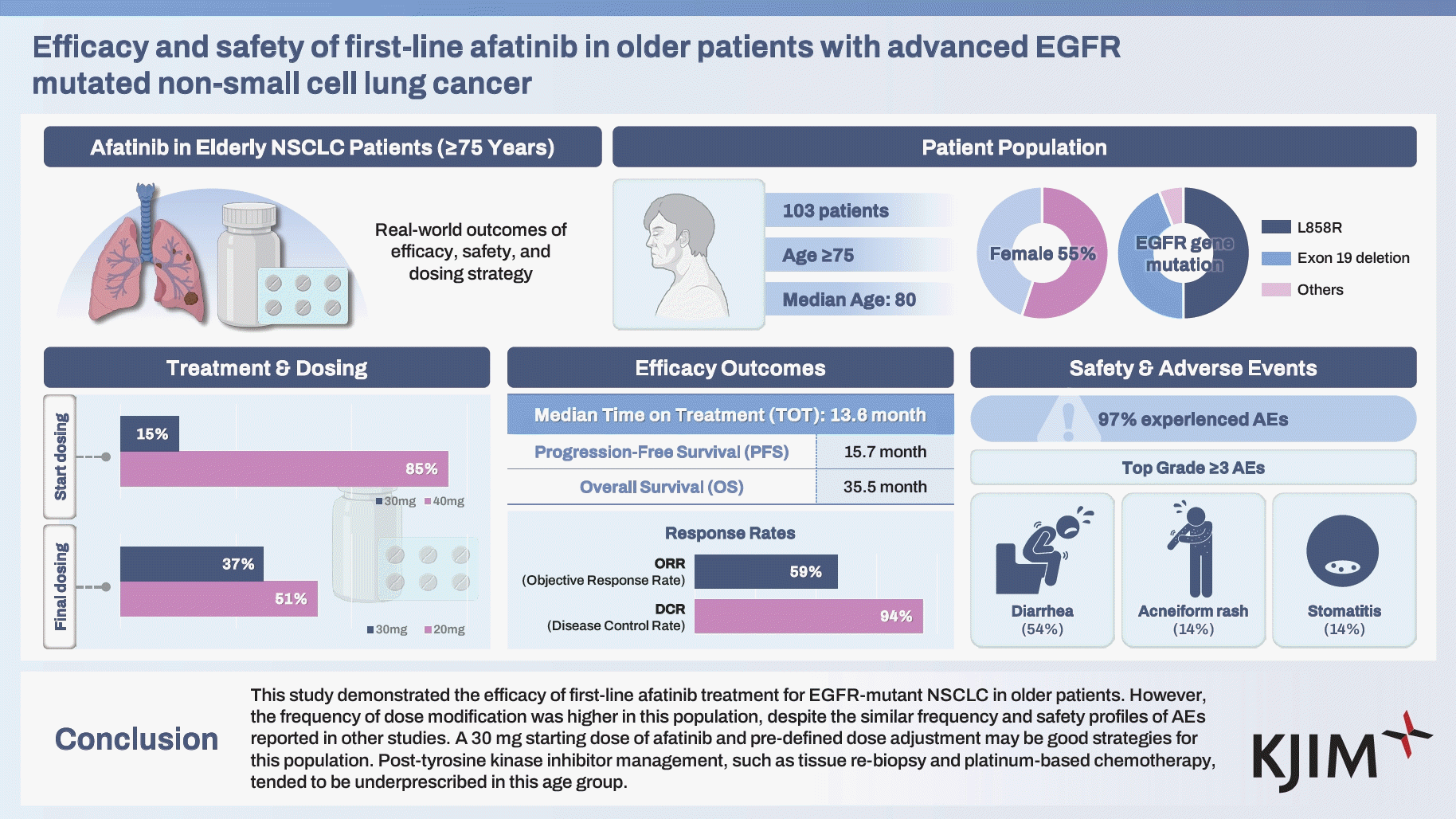
- This study investigated the efficacy and safety of first-line afatinib treatment in older patients with epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer (NSCLC).
- Methods
- This retrospective, multicenter, observational cohort study included 103 patients aged ≥ 75 years who were treated with first-line afatinib for EGFR-mutated NSCLC. The primary outcome was time-on-treatment (TOT).
- Results
- The median TOT of patients was 13.6 months (95% confidence interval 11.0–16.2). Ninety-two patients (89.3%) required dose modification. Dose reduction was significantly more frequent in the 40 mg starting dose group than in the 30 mg group (93.1% vs. 68.8%, p = 0.004). The most common grade 3 or worse adverse events (AEs) were diarrhea (n = 16, 54%), acneiform rash (n = 4, 14.3%), and stomatitis (n = 4, 14.3%). Grade 3 or worse AEs led to dose modification in 23 of 28 patients (82.1%) and permanent discontinuation of therapy in five of 28 patients (17.9%). On disease progression, tissue re-biopsy was performed in 18 of 74 patients (24.3%). Thirty-four patients (45.9%) received subsequent chemotherapy; of these, most patients (n = 21, 61.8%) received pemetrexed monotherapy.
- Conclusions
- This study demonstrated the efficacy of first-line afatinib treatment for EGFR-mutant NSCLC in older patients. However, despite similar safety profiles and frequencies of AEs reported in previous studies, the frequency of dose modifications was higher in this population. A 30 mg starting dose of afatinib and a predefined dose adjustment may be suitable strategies for this population. Post-tyrosine kinase inhibitor management, such as tissue re-biopsy and platinum-based chemotherapy, tended to be underprescribed in this age group.
- Graphical abstract
- Graphical abstract
- INTRODUCTION
- INTRODUCTION
Population aging is increasing more rapidly than in the past. According to the World Health Organization, the proportion of the world’s population aged > 60 years will nearly double from 12% to 22% between 2015 and 2050 [1]. In South Korea, lung cancer ranks third after thyroid and colorectal cancers; however, it is the most prevalent cancer type among individuals aged ≥ 65 years [2]. Moreover, 66.4% of patients newly diagnosed with lung cancer are aged ≥ 65 years in South Korea, which is consistent with global data [2,3].Osimertinib, a third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI), has been the preferred treatment regimen for advanced EGFR-mutant non-small cell lung cancer (NSCLC) since the FLAURA trial [4]. However, several first- and second-generation EGFR-TKIs, including afatinib, continue to be used as the first-line standard of care for many patients [5,6]. In addition, afatinib is the preferred option for uncommon EGFR mutations, such as S768I, L861Q, and G719X [6].Afatinib, an irreversible ErbB family blocker used as a second-generation EGFR TKI, has been approved for its efficacy in pivotal clinical trials of the LUX-Lung series [7-9]. The most common treatment-related adverse events (AEs) observed with afatinib include diarrhea, rash/acne, stomatitis/mucositis, and paronychia. Due to its irreversible binding to the EGFR, the rate of AEs for all grade AEs of afatinib is generally higher than that of first-generation EGFR TKIs [5]. Dose adjustments were recommended based on predefined tolerability criteria. A post-hoc analysis in the phase III LUXLung 3 trial showed that dose reductions occurred in 53% (122/229) of patients and the majority (86%) within the first six months of treatment [8,10]. Dose reduction tends to be more common in female patients, older patients, patients of Eastern Asian ethnicity, and patients with lower body weight [11].The number of older patients is increasing; however, no prospective EGFR-TKI trials have been conducted specifically in older patients. Therefore, subgroup analyses from prospective studies and extensive real-world data relative to this population are required. In this study, we investigated the efficacy and safety of first-line afatinib treatment in older patients with advanced EGFR-mutated NSCLC using real-world data.
- METHODS
- METHODS
- Study design and patient selection
- Study design and patient selection
This retrospective multicenter observational cohort study was conducted at three medical centers in South Korea. Electronic medical records from January 2014 to June 2022 of patients who met the following inclusion criteria were reviewed: (i) age ≥ 75 years with EGFR-mutant advanced-stage NSCLC and (ii) treatment with first-line afatinib. Advanced stages were defined as recurrent or inoperable stage III or IV disease based on the 8th Edition of the American Joint Committee on Cancer staging system.This study was approved by the Institutional Review Board (IRB) of Pusan National University Hospital (IRB no. 2307-001-128) and was conducted following the principles of the Declaration of Helsinki. The requirement for informed consent was waived by the IRB of the Pusan National University Hospital because of the retrospective nature of the study and because the analysis used anonymous clinical data.- Outcomes
- Outcomes
The primary outcome was time-on-treatment (TOT). TOT was defined as the time from the first afatinib dose to the last dose. Secondary outcomes included overall response rate (ORR), disease control rate (DCR), progression-free survival (PFS), and overall survival (OS). Response and progression were evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (version 1.1). PFS was calculated from the start of the first-line afatinib therapy to disease progression according to the RECIST guidelines (version 1.1) by the treating physician or death, whichever occurred earlier. OS was defined as the time from the initiation of first-line afatinib therapy to death from any cause.The safety data were descriptively analyzed. The incidence and severity of AEs, according to the Common Terminology Criteria for Adverse Events (version 6.0), when available, were summarized and displayed as numbers and percentages. For patients who underwent afatinib dose modifications, the frequency and severity of common AEs before and after dose modifications were analyzed.- Statistical analysis
- Statistical analysis
Categorical variables were analyzed using the χ2 test, Fisher’s exact test, or linear association test, as appropriate. Continuous variables were analyzed using the independent two-sample t-test, Mann–Whitney U test, or Wilcoxon signed-rank test. All time-to-event data relative to TOT, PFS, and OS were estimated using the Kaplan–Meier method, and the median and two-sided 95% confidence intervals (CIs) were calculated. All tests were two-sided, and statistical significance was set at p < 0.05. Statistical analyses were performed using SPSS version 26.0 (IBM Corp., Armonk, NY, USA).
- RESULTS
- RESULTS
- Patient and afatinib treatment characteristics
- Patient and afatinib treatment characteristics
A total of 103 older patients with advanced-stage EGFR-mutated NSCLC were enrolled in this study. The baseline characteristics of the study participants are summarized in Table 1. The median age was 80 years (75–91 years), and 57 patients (55.3%) were female. The L858R variant was the most common EGFR mutation (n = 51, 49.5%).The characteristics of afatinib treatment are shown in Table 2. A 40 mg or 30 mg starting dose of afatinib was allowed per the Korean National Health Insurance Service. In our study, most patients (n = 87, 84.5%) received a starting dose of afatinib of 40 mg. Baseline characteristics did not differ significantly according to the afatinib starting dose (Table 1). However, the frequency of dose reduction was significantly higher in the 40 mg starting dose group than in the 30 mg group (93.1% vs. 68.8%, p = 0.004). Fifty-three patients (51.5%) received a final afatinib dose of 20 mg/d, and 38 patients (36.9%) received 30 mg/d.- Efficacy outcomes
- Efficacy outcomes
The median TOT of the patients was 13.6 months (95% CI 11.0–16.2). No significant differences in efficacy outcomes were observed according to the starting dose of afatinib (Fig. 1). TOT was longer in patients with a 19del mutation (21.0 months, 95% CI 13.5–28.5) than in patients with an L858R mutation (13.6 months, 95% CI 11.9–15.3) or other mutations (8.3 months, 95% CI 12.9–13.7) (p = 0.001). The median PFS and OS of the study population were 15.7 (95% CI 11.2–20.2) and 35.5 months (95% CI 26.9–44.1), respectively. ORR and DCR were 59.2% and 94.2%, respectively.- Safety outcomes
- Safety outcomes
The safety data for all 103 patients are summarized in Table 3. Treatment-related AEs of any grade were reported in 100 patients (97.0%). The most common AEs were diarrhea (n = 57, 57.0%), acneiform rash (n = 44, 44.0%), stomatitis (n = 36, 36.0%), and paronychia (n = 20, 20.0%). Grade 3 or worse AEs were reported in 28 patients (27.2%) and included diarrhea (n = 16, 54%), acneiform rash (n = 4, 14.3%), and stomatitis (n = 4, 14.3%). Grade 3 or worse AEs led to a dose modification (dose interruption, dose reduction, or both) in 23 of 28 patients (82.1%) and permanent discontinuation of therapy in five of 28 patients (17.9%). After dose modification or permanent discontinuation of afatinib, most grade 3 or worse AEs recovered to grades 1 (n = 19) and 2 (n = 5), respectively.- Dose modification impact
- Dose modification impact
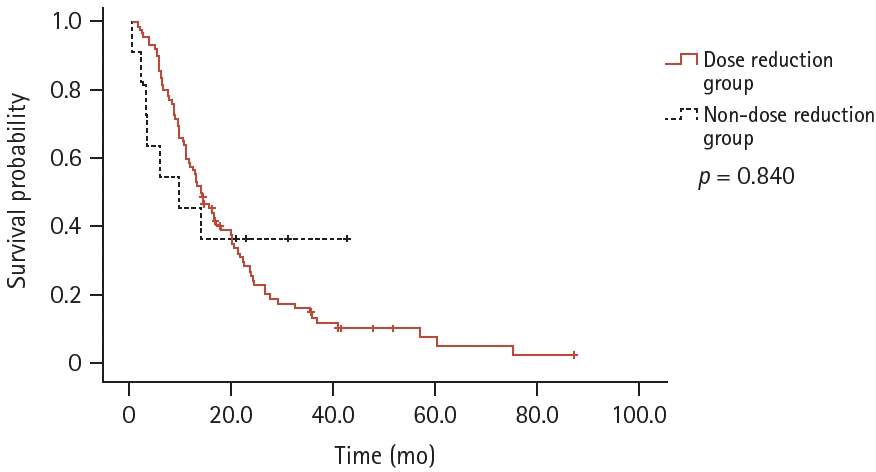
Ninety-two patients (89.3%) required dose modification. Among them, 47 required a second dose reduction, and 10 required an additional third dose reduction.In the dose reduction group, the ORR and DCR were 62.0% and 95.7%, respectively. In the non-dose reduction group, the ORR and DCR were 36.4% and 81.8%, respectively, with no statistical significance (p = 0.118 and p = 0.123, respectively). Moreover, the median TOT was 13.6 months (95% CI 10.6–16.6) in the dose-reduction group and 9.5 months (95% CI 0.0–21.0) in the non-dose reduction group (p = 0.840, Fig. 2).- Rebiopsy and subsequent treatment
- Rebiopsy and subsequent treatment
Seventy-four patients (71.8%) experienced disease progression during the study period. The re-biopsy results are shown in Table 4. Re-biopsy, including liquid biopsy, was performed in 49 samples from 43 of 74 patients (58.1%): 18 tissue samples (36.7%), 29 blood samples (59.2%), and 2 pleural fluid samples (4.1%). Six patients underwent EGFR tests in both tissue and blood samples. The T790M mutation was confirmed in nine patients (20.9%): six from tissues and three from blood samples.Thirty-four patients (45.9%) received chemotherapy. The second-line agents used are summarized in Table 5. Most patients (n = 21, 61.8%) received pemetrexed monotherapy. All nine patients with confirmed T790M mutations were treated with third-generation EGFR TKIs: osimertinib (n = 7) or lazertinib (n = 2). No significant differences were observed in survival according to subsequent therapy type.
- DISCUSSION
- DISCUSSION
In our study involving older patients, the median TOT was 13.6 months (95% CI 11.0–16.2), and the median PFS and OS were 15.7 (95% CI 11.2–20.2) and 35.5 months (95% CI 26.9–44.1), respectively. This result is consistent with that of previous prospective studies [7-9,12]. An observational real-world study recently described the efficacy of afatinib in older patients. The GIDEON study was conducted in Germany and involved patients with EGFR-positive NSCLC who received first-line treatment with afatinib [13]. The authors conducted a subset analysis of older patients (≥ 70 years of age) and reported the efficacy and safety of first-line treatment with afatinib compared with those of patients aged < 70 years. The median PFS and OS (17.2 and 30.4 months, respectively) in patients aged ≥ 70 years were similar to those in our study [14]. Therefore, the antitumor effects of afatinib are independent of age. In 2021, real-world data on afatinib was presented by 15 centers in South Korea, and the updated survival data of this study were published in 2023 (median PFS: 20.2 months, median OS: 48.6 months) [15,16]. In our study, we observed a median PFS of 15.7 months and an OS of 35.5 months. The potential reasons for these differences may include the larger cohort size (n = 422) used in the previous multicenter Korean study, with a mean age of 63.8 years [15,16]. Moreover, in the earlier study, the frequency of the 19del mutation, one of the most prominent prognostic factors for EGFR-TKIs, was 59.0% [15,16]. In contrast, our study focused on patients aged 75 years and older, with a mean age of 80 years, and the frequency of the 19del mutation was relatively lower at 43.7%. However, when analyzing efficacy outcomes specifically in patients with the 19del mutation in our study, we observed a PFS of 23.5 months and an OS of 35.5 months, which is comparable to the results from the multicenter Korean study. These findings suggest that factors such as age and mutation frequency influence survival outcomes.In this study, the median PFS (15.7 months) was slightly longer than the median TOT (13.6 months). This suggests that some cases of afatinib termination were due to toxicity or other reasons, despite which patients continued to have durable benefits, and in other patients, they were treated well beyond progression, based on the decision by the attending physicians, which likely contributed to the extension of TOT. This approach may have allowed continued clinical management even after disease progression, potentially explaining the relatively prolonged TOT compared to PFS.The frequency and severity of afatinib-related AEs in our study population were similar to those reported in previous studies [7-9,12]. However, the frequency of dose modification due to AEs was higher (89%) in this study than in the LUX-Lung 7 study, in which 42% of patients underwent dose modification [7,12]. Certain trials included an older cohort, such as the EURTAC trial [17] and the BR.21 study [18], which indicated that EGFR-TKIs improved survival but yielded a worse AE profile in older patients. In the older cohort of the BR.21 study, older patients experienced significantly more overall and severe (grade 3 and higher) toxicity than younger patients and were more likely to cease treatment owing to treatment-related AEs [19]. In South Korea, starting doses of afatinib of 40 and 30 mg are allowed in clinical practice. No significant differences in efficacy between the two starting doses were observed in this study; however, the frequency of dose reduction was significantly higher in the 40 mg starting dose group. Therefore, we suggest that a starting dose of 30 mg is a good treatment strategy for older patients.In the current study, re-biopsy was performed in 43 patients (58.1%); however, tissue re-biopsy was performed in only 18 patients (24.3%). Among the patients who underwent tissue re-biopsy, six patients (33.3%) harbored the T790M mutation. In only three patients (10.3%), T790M-positivity was detected among the 29 blood samples. This is consistent with previous data showing that plasma samples have a lower sensitivity for T790M mutation detection than tissue samples [20].Thirty-four patients (45.9%) received their anticancer therapy after discontinuation of afatinib. All patients with documented T790M mutations (n = 9) were treated with third-generation EGFR TKIs. Therefore, we suggest thorough tissue re-biopsy even in older patients. Among the 25 patients who received cytotoxic chemotherapy, 21 (84.0%) were administered pemetrexed monotherapy, whereas only four received platinum-based chemotherapy. In the FLAURA study, 48% of patients in the osimertinib group received subsequent treatment, and most patients (90/133) received chemotherapy. Among patients treated with chemotherapy, 93% (84/90) received platinum-containing chemotherapy [21]. Thus, older patients who were not positive for the T790M mutation tended to be undertreated, indicating that subsequent therapies may not provide a survival benefit in this population.This study has several limitations. First, this was a retrospective study with an inevitable bias. Second, this study had a relatively small number of enrolled patients, and certain patient characteristics, such as a relatively higher proportion of stage III and L858R mutations and initial brain metastases, could have influenced the results. Third, several factors influencing the decisions of the physician, such as the starting dose of afatinib, may have played a role in patient management. Fourth, although 75 years of age was chosen as the reference point in this study, the optimal reference point for older patients remains unclear. Finally, the frequency and severity of afatinib-related AEs in our older study population were similar to those of previous prospective studies that did not target older patients, which might be underestimated owing to the retrospective nature of the study.In conclusion, this study supports the efficacy of first-line afatinib treatment for EGFR-mutant NSCLC in older patients. However, the dose modification frequency was higher in this population despite the similar frequency and safety profiles of AEs reported in other studies. A 30 mg starting dose of afatinib and a predefined dose adjustment may be suitable strategies for the older population. Post-TKI management, such as tissue re-biopsy and platinum-based chemotherapy, tended to be underprescribed in this age group. Proactive management is considered necessary, particularly for older patients with good performance status.
- KEY MESSAGE
- KEY MESSAGE
1. First-line afatinib is an effective treatment option for older patients.2. Post-TKI management, including tissue re-biopsy and platinum-based chemotherapy, tended to be underprescribed in this age group.3. A 30 mg starting dose of afatinib and a predefined dose adjustment may be good strategies for this population.
Figure 1.
Survival outcomes of all patients according to the afatinib starting dose. Time-on-treatment (A), progression-free survival (B), overall survival (C).
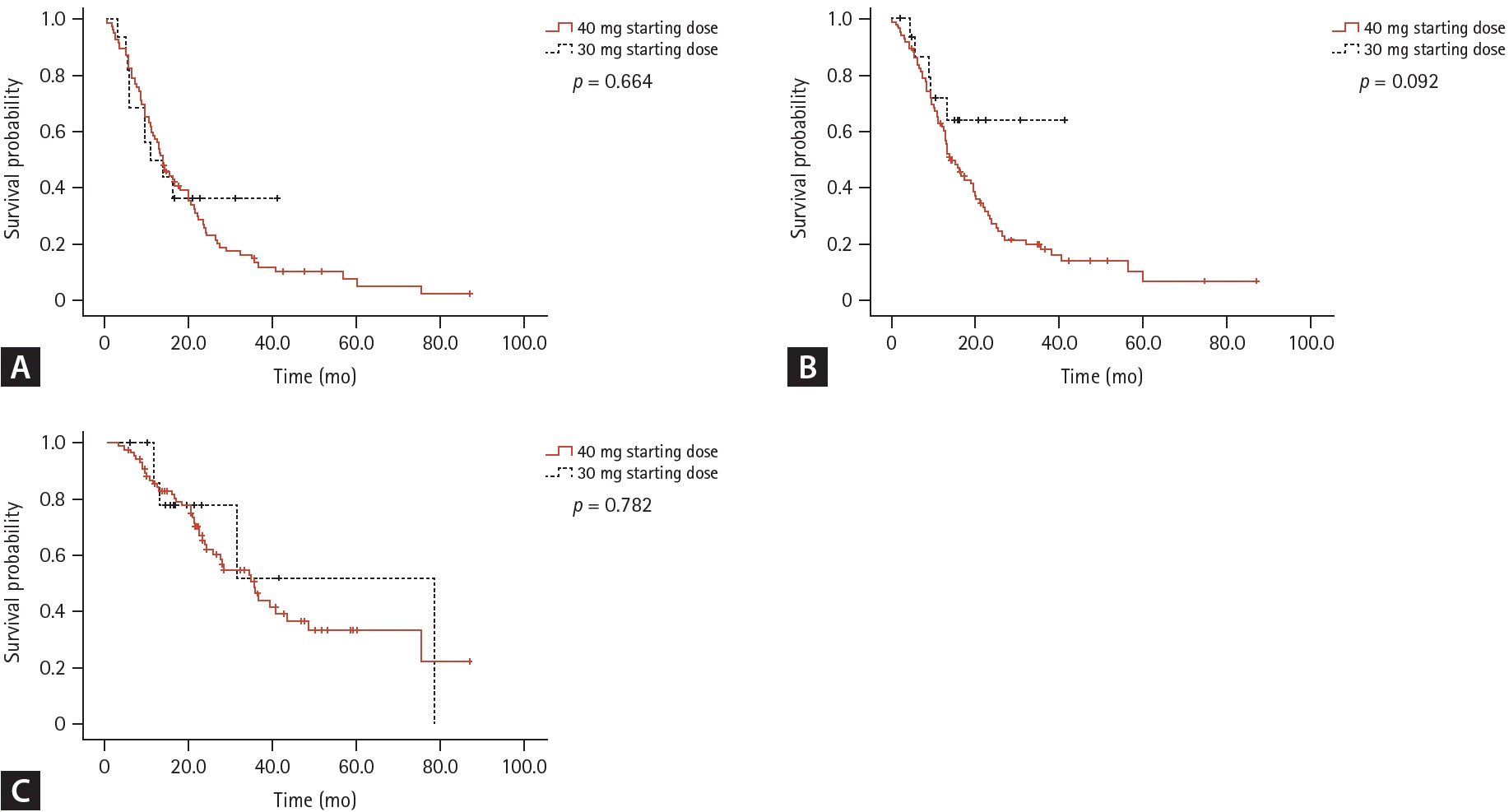
Table 1.
Patient characteristics (n = 103)
| Characteristic | Value | 40 mg starting dose (n = 87) | 30 mg starting dose (n = 16) | p value |
|---|---|---|---|---|
| Age (yr) | 80 (75–91) | 81 (75–91) | 79 (76–86) | 0.219 |
| Sex, female | 57 (55.3) | 47 (54.0) | 10 (62.5) | 0.594 |
| Never smoker | 64 (62.1) | 54 (62.1) | 10 (62.5) | 0.941 |
| ECOG PS 0-1 | 89 (86.4) | 74 (85.1) | 15 (93.8) | 0.317 |
| Histology | 0.682 | |||
| Adenocarcinoma | 99 (96.1) | 83 (95.4) | 16 (100.0) | |
| Squamous cell carcinoma | 3 (2.9) | 3 (3.4) | 0 (0.0) | |
| Non-small cell lung cancer, not otherwise specified | 1 (1.0) | 1 (1.1) | 0 (0.0) | |
| Stage | 0.093 | |||
| III | 14 (13.6) | 13 (15.0) | 0 (0.0) | |
| IV | 89 (86.4) | 73 (83.9) | 16 (100.0) | |
| Initial EGFR mutation type | 0.857 | |||
| 19del | 45 (43.7) | 37 (42.5) | 8 (50.0) | |
| L858R | 51 (49.5) | 44 (50.6) | 7 (43.8) | |
| Othersa) | 7 (6.8) | 6 (6.9) | 1 (6.3) | |
| Initial brain metastasis | 33 (32.0) | 28 (32.2) | 5 (31.3) | 0.397 |
| Number of distant metastasis | 1 (0–7) | 1 (0–7) | 2 (1–6) | 0.226 |
Table 2.
Afatinib exposure
| Characteristic | Value (n = 103) |
|---|---|
| Initial dose | |
| 40 mg | 87 (84.5) |
| 30 mg | 16 (15.5) |
| Dose reduction frequency | 92 (89.3) |
| Final dose | |
| 40 mg | 6 (5.8) |
| 30 mg | 38 (36.9) |
| 20 mg | 53 (51.5) |
| 20 mg every other day | 4 (3.9) |
| Permanent stop | 2 (1.9) |
| Reason for stop of afatinib (n = 84) | |
| Disease progression | 52 (61.9) |
| Adverse events | 9 (10.7) |
| Patient’s wish | 3 (3.6) |
| Follow-up loss | 6 (7.1) |
| Othera) | 14 (16.7) |
Table 3.
Adverse events
Table 4.
Re-biopsy epidermal growth factor receptor test results (n = 49)
| Tissue (n = 18) | Blood (n = 29) | Pleural effusion (n = 2) | |
|---|---|---|---|
| Only sensitive | 11 (61.1) | 13 (44.8) | 1 (50.0) |
| Sensitive/T790M | 6 (33.3) | 3 (10.3) | 0 (0.0) |
| Not detected | 1 (5.6) | 13 (44.8) | 1 (50.0) |
Table 5.
Second-line agents (n = 34)
| Agent | Value |
|---|---|
| Pemetrexed monotherapy | 21 (61.8) |
| Pemetrexed-platinum | 2 (5.9) |
| Gemcitabine-platinum | 2 (5.9) |
| Osimertinib | 7 (20.6) |
| Lazertinib | 2 (5.9) |
- References
- References
REFERENCES
1. World Health Organization. Ageing and health [Internet]. Geneva (CH): World Health Organization, c2024 [cited 2024 Jul 22]. Available from: https://www.who.int/news-room/factsheets/detail/ageing-and-health.2. Korea Central Cancer Registry, National Cancer Center. Annual report of cancer statistics in Korea in 2021 [Internet]. Sejong (KR): Ministry of Health and Welfare, c2023 [cited 2024 Jul date]. Available from: https://ncc.re.kr/cancerStatsView.ncc?bbsnum=678&searchKey=total&searchValue=&page-Num=1.3. National Cancer Institute. Cancer stat facts: lung and bronchus cancer [Internet]. Maryland (US): National Cancer Institute, c2024 [cited 2024 Jul 22]. Available from: https://seer.cancer.gov/statfacts/html/lungb.html.4. Soria JC, Ohe Y, Vansteenkiste J, FLAURA Investigators, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018;378:113–125.
[PubMed]5. Stinchcombe TE. Flashback foreword: afatinib for the treatment of epidermal growth factor receptor mutation-positive non-small-cell lung cancer. J Clin Oncol 2023;41:2867–2868.
[Article] [PubMed]6. National Comprehensive Cancer Network. NCCN guidelines version 5 [Internet]. Plymouth Meeting (PA): National Comprehensive Cancer Network, c2024 [cited 2024 Jul 22]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.7. Park K, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016;17:577–589.
[Article]8. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327–3334.
[Article] [PubMed]9. Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213–222.
[Article] [PubMed]10. Yang JC, Sequist LV, Zhou C, et al. Effect of dose adjustment on the safety and efficacy of afatinib for EGFR mutation-positive lung adenocarcinoma: post hoc analyses of the randomized LUX-Lung 3 and 6 trials. Ann Oncol 2016;27:2103–2110.
[Article] [PubMed]11. Yang JCH, Ahn MJ, Dickgreber NJ. Influence of dose adjustment on afatinib safety and efficacy in patients (pts) with advanced EGFR mutation-positive (EGFRm+) non-small cell lung cancer (NSCLC). J Clin Oncol 2015;33(15_suppl):8073–8073.
[Article]12. Paz-Ares L, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-smallcell lung cancer: overall survival data from the phase IIb LUXLung 7 trial. Ann Oncol 2017;28:270–277.
[Article] [PubMed] [PMC]13. Brückl WM, Reck M, Griesinger F, et al. Afatinib as firstline treatment in patients with EGFR-mutated non-small cell lung cancer in routine clinical practice. Ther Adv Med Oncol 2021;13:17588359211012361.
[PubMed] [PMC]14. Brueckl WM, Reck M, Schäfer H, et al. Older patients with EGFR mutation-positive non-small cell lung cancer treated with afatinib in clinical practice: a subset analysis of the non-interventional GIDEON study. J Geriatr Oncol 2023;14:101394.
[Article] [PubMed]15. Lee SY, Choi CM, Chang YS, et al. Real-world experience of afatinib as first-line therapy for advanced EGFR mutation-positive non-small cell lung cancer in Korea. Transl Lung Cancer Res 2021;10:4353–4367.
[Article] [PubMed] [PMC]16. Choi J, Choi CM, Chang YS, et al. Real-world first-line afatinib for advanced EGFR mutation-positive non-small cell lung cancer in Korea: updated survival data. Transl Lung Cancer Res 2023;12:2275–2282.
[Article] [PubMed] [PMC]17. Rosell R, Carcereny E, Gervais R, Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239–246.
[PubMed]18. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, National Cancer Institute of Canada Clinical Trials Group, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123–132.
[Article] [PubMed]19. Wheatley-Price P, Ding K, Seymour L, Clark GM, Shepherd FA. Erlotinib for advanced non-small-cell lung cancer in the elderly: an analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol 2008;26:2350–2357.
[Article] [PubMed]