 |
 |
| Korean J Intern Med > Volume 10(1); 1995 > Article |
|
Abstract
Objectives
Small cell lung cancer is sensitive to chemotherapy and radiotherapy. Nevertheless, responses are still short-lived and apparent cure remains for only limited disease patients.
Methods
We combined cyclophosphamide (750mg/m2 by intravenous infusion at first day) vincristine (2mg intravenously at third day), cisplatin (20mg/m2 intravenously for 3 days), and etoposide (100mg/m2 intravenously for 3 days) with radiotherapy (total 300cGy over 4 weeks in 17 fractions) and treated 39 patients with small cell lung cancer who had received no prior systemic chemotherapy and radiotherapy.
Results
Thirty-nine patients (limited disease: 17 patients, extensive disease 22 patients) were treated and 35 patients were evaluable for response. Overall response rate was 82.8% (complete response 28.6%, partial response 54.2%)
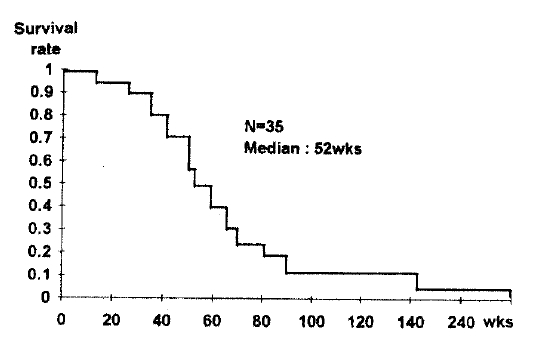
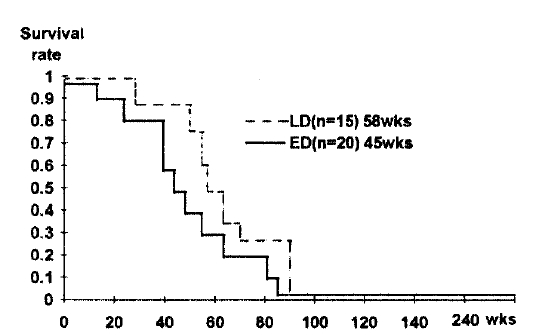
The median survival was 52 weeks for all patients and 58 weeks for limited disease and 45 weeks for extensive disease. There was no statically significant survival difference between the two patient groups. The median relapse-free survival time was 48 weeks.
Overall, treatment was well tolerated, with granulocytopenia being the most frequent toxicity.
Small cell lung cancer (SCLC) comprises 20% to 25% of all the lung cancers and differs from the other histologic types of lung cancer with respect to its biologic behavior and its responsiveness to chemotherapeutic agents and radiotherapy. The natural history of small cell lung cancer is characterized by rapid clinical course and dissemination, and by its sensitivity to available drugs1,2). This disease is rapidly fatal with a median survival of 5ŌĆō7 weeks in extensive disease and 12 weeks in limited disease in untreated patients3). On the other hand, chemotherapy, radiotherapy or both are capable of producing responses in 60ŌĆō90% of patients with significant improvemint in survival4,5). Nevertheless, responses are still short-lived and apparent ŌĆ£cureŌĆØ remains for only limited disease patients.
Although many combinations of chemotherapeutic agents for small cell lung cancer appear to yield similar therapeutic results, CAV (cyclophosphamide, doxorubicin, vincristine) has become one of the most commonly used regimens6). Many attempts have been made to increase therapeutic results either by addition of other drugs or by individual replacement. Recently, the combination of VP-16 and cisplatin has been shown to be as effective as or slightly superior to CAV and partially non-cross resistent7). Cyclophosphamide is known as one of the most effective single agents in the management of SCLC 8). Additionally, cyclophosphamide is synergistic with VP-16 and cisplatin9). The combination of 3 drugs (cyclophosphamide, cisplatin, VP-16) was tested by Eagan et al in a small pilot study10). Eleven patients with limited disease achieved 4 complete responses and 5 partial responses with a median survival of 53 weeks. Thirteen patients with extensive disease achieved 4 complete responses and 1 partial response. Vincristine is known to be an active single agent11) and has been a frequent component of highly effective drug combinations, such as with cyclophosphamide12). Therefore we attempted to enhance the therapeutic efficacy of VP-16 and cisplatin by the addition of cyclophosphamide and vincristine (COPE). After chemotherapy-induced complete remission, the chest and central nervous system remain the significant sites of failure in patients with limited disease7).
We, therefore, reviewed retrospectively our experience with patients treated during the past 4 years with combination chemotherapy (COPE) combined with chest irradiation and prophylactic cranial irradiation who had previously untreated small cell lung cancer. Our aims were as follows: (1) to evaluate the overall survival and disease-free survival. (2) to evaluate their toxicities.
Patients were eligible for the study if they had histologically confirmed small cell lung cancer, no previous chemotherapy or radiotherapy, performance status of 0ŌĆō3 on the ECOG scale, age <70years and no previous severe underlying medical illnesses. All patients enrolled were required to have adequate leukocyte count (>4.0├Ś103/mm3), platelet count (>100├Ś103/mm3), renal function (serum creatinine <1.5mg/dl) and hepatic function (serum bilirubin < 3mg/dl).
Initial diagnostic work included history taking and physical examination, CBC, blood chemistry and urine examination for all patients. The pretreatment staging was usually determined by physical examination, chest radiography, whole-lung computerized tomography, fiberoptic bronchoscopy, ultrasonography or computed tomography of the abdomen, bone marrow examination and bone scintiscan if skeletal sympotoms were present. CBC was reperted before each cycle of chemotherapy and also 14 days after each course.
Staging was done by a two-stage system based on the above examinations: limited disease (LD) was defined as disease confined to one hemithorax and regional lymph nodes, including the bilateral mediastinal, contarlateral hilar and ipsilateral supraclavicular nodes, while extensive disease (ED) was defined as disease beyond this and included malignant pleural effusion.
Chemotherapy was started immediately after diagnosis was obtained and consisted of cyclophosphamide 750mg/m2 on day 1; vincristine 2. Omg on day 3; cisplatin 20mg/m2 on day 1, 2 and 3; etoposide 100mg/m2 on day 1, 2 and 3. The courses were repeated every three weeks until 6 cycles (Table 1). After 6 cycles of chemotherapy were completed, thoracic radiotherapy was done to all responders, regardeless of stage. The total tumor dose was 300cGy over 4 weeks in 17 fractions. Prophylactic cranial irradiation (PCI) was given only to patients in complete response after completion of all chemotherapy. PCI (300 cGy) was given in 10 fractions.
The dose of cyclophosphamide, cisplatin and etoposide were modified according to white blood cell (WBC) count, checked before the start of each cycles.
Objective response was determined according to the criteria of WHO13); complete remission (CR), partial remission (PR), stable disease (SD) and progressive disease (PD), and it was based primarily on chest radiography and clinical findings. The response rate included an complete and partial response that persists during at least 1 month. Overall survival was calculated as the period from the commencement of chemotherapy to the day of death. For complete and partial responders, duration of response or relapse-free survival was calculated as the period from the first day of treatment to the day when progressive disease was first observed.
Acute and subacute toxicities were evaluated according to the criteria of WHO based on blood cell counts14).
The Kaplan-Meier method was used to estimate the survival distribution, and log-rank test and chi-square test were used to evaluate differences in survival and relapse-free survival.
Among thirty-nine patients with histologically proven SCLC from June 1989 to 1993 at Korea University Hospital, thiry-five patients who received at least two courese of chemotherapy were considered evaluable for response.
Table 2 lists patient characteristics: 29 (83%) were men and 6 (17%) were women. The median age was 61 years (range, 33ŌĆō70years) at the start of therapy. Of the 35 patients assigned a performance status (PS) (ECOG scale), 12(34%) were classified as PS 0 or 1.18 (52%) as PS 2, and 5 (14%) as PS 3.
The overall response rate was 83%, with 29% CR and 54% PR. Among 15 patients with LD, 6 patients (40%) showed CR, 7 patients (47%) PR, and 2 patients (13%) no response. Details of response by stage and response rate are given in Table 3.
The median survival time for all 35 patients from the first treatment with COPE was 52 weeks (range 16ŌĆō235weeks). Survival is shown in Fig. 1. In patients with LD, the median survival time was 58 weeks and in patients with ED it was 45 weeks (Fig 2). Although median survival time was longer in LD patients, there was no statistically significant difference in the two patient groups (p = 0.12). In response rate, according to tumor response, median survival was 52 weeks in responders (CR:62 weeks PR:51 weeks) and 44 weeks in non-responders (SD + PD).
Relapse-free survival was evaluated for all 29 responders and median relapse-free survival time was 48weeks. Although there was no statistically significant difference in the two patient groups, it was longer in patients with LD.
Toxicity was evaluable in all 35 patients. Leukopenia was the major dose-limiting side-effect. Eleven patients (31.5%) had leukopenia of WHO grade 3 or 4. Details are shown in Table 4. Alopecia of grade 2 to 3 was seen in almost all patients. Nausea and vomiting were generally mild to moderate and managed with antiemetics. Grade 2 hepatotoxicity and grade 1 azotemia were seen in 1 and 2 patients, respectively.
The introduction of CAV (cyclophosphaminde, doxorubicin, vincristine) chemotherapy induced a high incidence of complete remissoin and a prolongation of median survival. Most studies reported 70 to 100% of overall response, 40 to 60% of CR in patients with LD, and 50 to 90% of overall response, 25% of CR incidence in patients with ED. Most studies also report a median survival of 48 to 56 weeks for LD and 24 to 32 weeks for ED4,5). In our study evaluating the efficacy of COPE regimen, we observed that an objective response rate was 83% (CR: 29%, PR: 54%). Among these, CR rate was 40% and PR rate was 47% in patients with LD and the rate of CR was higher than that of ED patients (CR: 20%, PR: 60%). These results compare favorably with complied data from the literatures with the CAV or other combinations. In a recently published Japanese study15), alternating CAV-PE was compared with CAV and PE. The response rate for PE (78%) and CAV-PE (76%) was significantly higher than the rate of CAV (55%). In the study of Evans by VP-16 and cisplatin, there was an overall response rate of 86% and median survival time of 70 weeks in LD patients and 43 weeks in ED patients. Additionally, in the study of Eagan10) evaluating the efficacy of cyclophosphamide, cisplatin, and VP-16, the overall response rate was 79%. In that study, overall response rate and CR rate were 82% and 36%, respectively, in LD patients, and 77%, 31% in ED patients. In studies using other regimens in Korea16,17,18), they reported 70 to 80% of overall response rate, 35 to 55 weeks of median survival in LD and ED patients with small cell lung cancer. Independent of disease stage, performance status is one of the most powerful independent prognostic factors19). In our study, the performace score of 23 patients (66%) was 2ŌĆō3 on the ECOG scale. Therefore, it is suspected that the effect of combination chemotherapy with COPE is as effective or slightly superior to the effect of other regimens used in some other Korean studies because 13ŌĆō37% of patients enrolled had a performance score of 2ŌĆō3 in two studies in Korea (Table 5).
In toxicity, leukopenia was the major dose-limiting side-effect, and dose reduction was needed in 6 patients. Most patients suffered from nausea, vomiting, alopecia, but these symptoms were generally mild to moderate and controlled with antiemetics.
Radiation therapy has long been a major tool in the treatment of limited-stage small cell lung cancer. Several prospective randomized trials reported significantly improved overall survival (extension of median survival by 2 to 4 months) and 2-year disease free survival with combined regimens, regardless of time of radiation therapy, such as concurrent or sequential irradiation.
In a large retrospective review of the literature, the addition of chest irradiation to chemotherapy for patients with extensive stage SCLC reduced frequency of local chest recurrence, but did not alter the objective response rates, median survival20).
Optimal methods for combining chemotherapy and chest radiotherapy are still on debate. Compared with concurrent chemo and radiotherapy, alternating or interdigitating regimens appear to have reduced pulmonary toxicity, while maintaining therapeutic efficacy21).
Acturial analysis reveals a probability of brain metastasis approaching 50% to 80% in 2-year survivors without prior therapy to the central nervous system22). In a review of 702 patients, initially free of clinical brain metastasis, prophylactic cranial irradiation (PCI) reduced the frequency of clinical brain metastasis from 20% to 6%. However there was no significant impact on survival associated with prophylactic cranial irradiation23).
In summary, our data indicate that combination chemotherapy with COPE regimen combined with radiation therapy was as effective as other studies and useful as a first line therapy for small cell lung cancer (Table 5). Further comparative study will be needed with CAV or PE regimens.
Table┬Ā1.
Dose and Schedule of COPE Chemotherapy
| Day | 1 | 2 | 3 | 22 |
|---|---|---|---|---|
| Cyclophosphamide | * | |||
| (750mg/m2) | ** | |||
| Vincristine (2mg) | * | |||
| Cisplatin (20mg/m2) | * | * | * | |
| Etoposide (100mg/m2) | * | * | * |
Table┬Ā2.
Characteristics of Patients with Small Cell Lung Cancer
Table┬Ā3.
Response of Therapy
| Response | LD (%) | ED (%) | Overall (%) |
| CR | 6/15 (40) | 4/20 (20) | 10/35 (28.6) |
| PR | 7/15 (47) | 12/20 (60) | 19/35 (54.2) |
| SD | 2/15 (13) | 1/20 (5) | 3/35 (8.6) |
| PD | 3/20 (15) | 3/35 (8.6) |
Table┬Ā4.
Toxicity
REFERENCES
1. Kim HJ, Jeong MP, Heo DS, Bang YJ, Han SK, Shin YS, Kim NK, Kim KY. Lung cancer in Korea (1980ŌĆō1984). Korean J Intern med 46(2):221ŌĆō2281994.
2. Cohen MH, Matthews MJ. Small cell bronchogenic carcinoma: A distinct clinicopathologic entity. Semin Oncol 5:234ŌĆō2431978.


3. Zelen M. Keynote address on biostatistics and data retrieval. Cancer Chemother Rep 4:31ŌĆō421973.
4. Cohen MH, Creaven PJ, Fossieck BE, Broder LE, Selawry OS, Johnston AV, Williams CL, Minna J. Intensive chemotherapy of small cell bronchogenic carcinoma. Cancer Treat Rep 61:349ŌĆō3541977.

5. Holoye PY, Samuels ML, Lanzotti VJ, Smith T, Barkley HT Jr. Combination chemotherapy and radiation therapy for small cell carcinoma. JAMA 237:1221ŌĆō12241977.


6. Greco FA, Richardon RL, Snell JD, Stroup SL, Oldham RK. Small cell lung cancer: Complete remission and improved survival. Am J Med 66:625ŌĆō6301979.


7. Evans WK, Shepherd FA, Feld R, Osoba D, DeBoer G. First-line therapy with VP-16 and cisplatin for small cell lung cancer. Semin Oncol 13(3):17ŌĆō231986.


8. Selawry OS. Monotherapy of bronchogenic carcinoma with special reference to cell type. Cancer Chemother Rep(Part 4) 4(2):177ŌĆō1881973.
9. Gale GR, Atkins LM, Meischen SJ, Smith AB, Walker EM Jr. Combination chemotherapy of LI210 leukemia with platinum compounds and cyclophosphamice plus other selected antineoplastic agents. N NatŌĆÖl Cancer Inst 57:1363ŌĆō13661976.

10. Eagan RT, Frytak S, Nichols WC, Ingle JN, Creagan ET, Kvols LK. Cyclophosphamide and VP-16-213 with or without cisplatin in squamous cell and small cell lung cancers. Cancer Treat Rep 65(506):453ŌĆō4581981.

11. Dombernowsky P, Hansen HH, Sorensen PG, Hainau B. Vincristine(NSC-67574) in the treatment of small cell anaplastic carcinoma of the lung. Cancer Treat Rep 60(3):239ŌĆō2421976.

12. Holoye PY, Samuels ML. Cyclophosphamide, vincristine and sequential split-course radiotherapy in the management of small cell lung cancer. Chest 67:675ŌĆō6791975.


13. WHO. Handbook in reporting results on cancer treatment. WHO Offset Publication No. 48. Geneva: World Health Organization, 1979.
14. Miller AB, Hoogstraten B, Staquet M, Winker A. Reporting results of cancer treatment. Cancer 47:207ŌĆō2141981.


15. Fukuoka M, Furuse K, Saijo N, Nishwaki Y, Ikegami H, Tamura T, Shimoyama M, Suemasu K. Randomized trial of cyclophosphamide, doxorubicin and vincristine versus cisplatin, and etoposide versus alternation of these regimens in small cell lung cancer. J Natl Cancer Inst 83:855ŌĆō611991.


16. Kwon SI, Kim JH, Kim JS, Uh WG, Kim SY, Yoon HJ, Cho KS. Cispaltin and etoposidel (VPP) combination chemotherapy in small cell lung cancer. Korean J of Intern Med 44(2):228ŌĆō2371993.
17. Sohn CH, Lee BC, Shin HK, Cho KJ. Alternating non-cross-resistent chemotherapy with CAV (cyclophosphamide, adriamycin, vincristine) and EP (etoposide, cisplatin) in small cell lung cancer. J Korean Cancer Association 24(4):570ŌĆō5761992.
18. Bang YJ, Park SY, Kim NK, Sim YS, Kim KY, Han YC, Ha SW, Park CI. Combination chemotherapy with VP-16, itostamide and epirubicine (VIE) for small cell lung cancer. J Korean Med Assoc 31(2):212ŌĆō2191988.
19. Abrams J, Doyle LA, Aisner J. Staging, Prognostic factors, and special considerations in small cell lung cancer. Semin Oncol 15(3):261ŌĆō2771988.

20. Lichter AS, Bunn PA, Ihde DC, Cohen MH, Makuch RW, Carney DN, Johnston-Early A, Minna JD, Glatstein E. The role of radiation therapy in the treatment of small cell lung cancer. Cancer 55:2163ŌĆō21751985.


21. Arrigada R, LeChevalier T, Baldeyrou P, Pico JL, Ruffie P, Martin M, EIBakry HM, Duroux P, Bignon J, Lenfant B, Hayat M, Rouesse JG, Sancho-Garnier A, Tubiana M. alternating radiotherapy and chemotherapy schedules in small cell lung cancers, limited disease. Int J Rad Oncol Biol Phys 11:1461ŌĆō14671985.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



