 |
 |
| Korean J Intern Med > Volume 11(2); 1996 > Article |
|
Abstract
We describe a 17-year-old male who presented with thrombotic thrombocytopenic purpura (TTP) and 2 years thereafter developed central nervous system lupus and nephritis. The association of TTP and systemic lupus erythematosus has been described, but the unusual sequence and chronological separation is very rare.
Thrombotic thrombocytopenic purpura (TTP) is a rare clinical syndrome of unknown etiology and characterized by the presence of microangiopathic hemolytic anemia, thrombocytopenic purpura, neurologic abnormalities, fever and renal dysfunction1,2). TTP associated with systemic lupus erythematosus (SLE) has been described on other occasions, but mostly the diagnosis of SLE preceded or coincided with that of TTP3ŌĆō11). We report a case of SLE that developed 2 years after the complete resolution of TTP, emphasizing the unusual sequence and the chronological separation of the two diseases.
A 17-year-old male was admitted due to fever and aphasia. He was in good health until 1 week before admission when he developed a sore throat, fever up to 38┬░C and global aphasia one day prior to admission. The past medical history was unremarkable but his cousin was diagnosed to have systemic lupus erythematosus.
On examination, blood pressure was 140/90 mmHg, heart rate was 120/min, respiration rate 22/min and temperature 38.0┬░C. There was no rash or petechiae. The head and neck were normal, the conjunctivae were slightly anemic. The lungs were clear and the heart sound was regular without murmur. Abdominal examination showed no organomegaly. Neurological examination was remarkable only for global aphasia.
Laboratory data revealed a hemoglobin level of 10.8 g/dl, hematocrit 32.5%, white blood cell count 4,600/mm3 with 62% segmented neutrophils, 23% lymphocytes, 9% monocytes, 4% metamyelocytes and 2% band neutrophils. Platelet count was 8,000/mm3. The blood smear revealed several fragmented red cells and scarcity of platelets (Fig. 1). Reticulocyte count was 7.9%. The urinalysis showed 1+protein, 1ŌĆō3 white cells and moderate blood cells/high power field. The fasting blood sugar was 96 mg/dl, the urea nitrogen 16 mg/dl, the creatinine 1.5 mg/dl, the total protein 7.4 g/dl and the albumin 4.6 g/dl. The total bilirubin was 3.4 mg/dl and direct bilirubin 0.6 mg/dl, lactate dehydrogenase was 1,020 IU/l. Prothrombin time was 70% (1.21 INR), partial thromboplastin time 28.9 s (control 28.9 s), fibrinogen 435 mg/dl (normal 200ŌĆō400 mg/dl), fibrin split product 10 (normal<10) and D-dimer was negative. Direct and indirect CoombsŌĆ▓ tests were negative, serum complement levels were within normal range. Anti-ds-DNA antibody was 4.5 IU/ml (normal<7 IU/ml). FANA was negative. Cultures from blood and urine did not show pathogenic organism.
On the 3rd day of admission, bone marrow aspiration and biopsy showed endothelial cell proliferation of vessels and microthrombi in the lumen consistent with the diagnosis of TTP (Fig. 2).
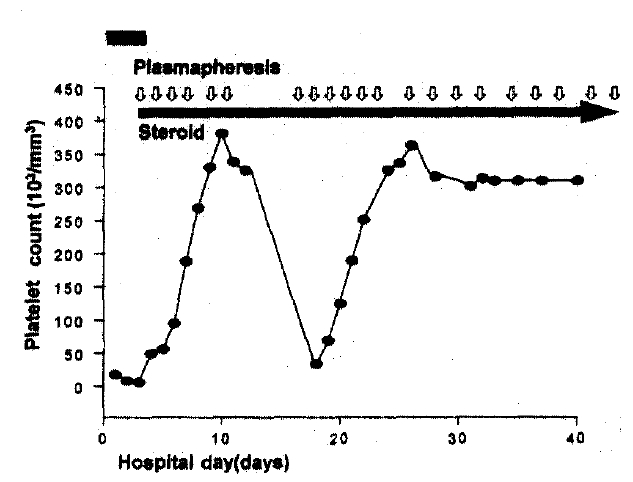
Treatment with oral prednisolone (60 mg/day) and plasmapheresis started on the 3rd day. Aphasia gradually improved, and after 21 sessions of plasmapheresis lasting 36 days (10ŌĆō20 units of fresh frozen plasma replacement per session) he fully recovered from neurologic deficit and became afebrile (Fig. 3). At discharge, laboratory findings showed hemoglobin 14.0 g/dl, hematocrit 41.1%, WBC 9,300/mm3, platelets 339,000/mm3, reticulocyte count 2.0%, serum creatinine 0.3 mg/dl, total bilirubin 0.3 mg/dl and lactate dehydrogenase 406 IU/l. Oral prednisolone was tapered off during the next eight months.
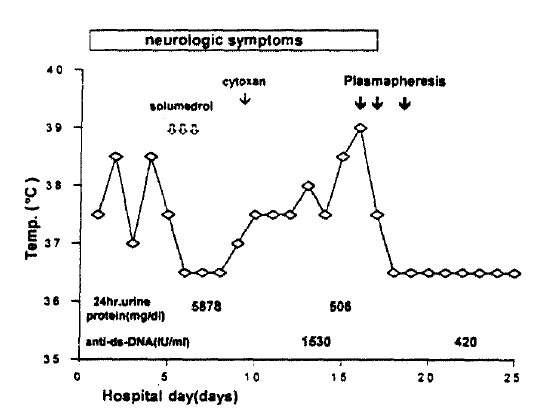
Two years after his first admission, the patient presented with fever of one month duration and generalized tonic-clonic seizure that developed one day prior to admission. He also complanined of 14 kg weight loss for the preceding 5 months, headache, alopecia, oral ulceration and vomiting. Physical examination revealed no remarkable finding, except irritability and decreased cognitive function. Laboratory findings showed hemoglobin 9.7 g/dl, hematocrit 27.6%, WBC 3,800/mm3 with 72% segmented neutrophils, 23% lymphocytes, 3% monocytes and 1% eosinophils, platelet count 196,000/mm3, erythrocyte sedimentation rate 35 mm/h, creatinine 1.0 mg/dl, the total protein 6.7 g/dl, the albumin 3.0 g/dl, bilirubin 0.4 mg/dl and LDH 449 IU/l. The 24 hour urinary protein quantitation showed 5,878 mg/day. C3 level was 44 (normal 85ŌĆō193), C4 less than 8 (normal 12ŌĆō36), and total hemolytic complement (CH50) was 28 (normal 100ŌĆō300). Serum IgG was 2,260 mg/dl (normal 63ŌĆō277). FANA test was positive at a titer of 1:640 with a speckled pattern, and anti-RNP and anti-Sm anti-bodies were also positive. Anti-ds-DNA antibody was elevated upto 1,530 IU/l (normal<7 IU/l). Anticardiolipin antibodies and lupus anticoagulant were negative.
Spinal puncture disclosed a normal opening pressure and lymphocytosis of 35/mm3, with clear fluid; pH 8.2; glucose 47 mg%; protein 59 mg%. MRI of the brain and echocardiography showed no abnormal finding. A renal biopsy revealed diffuse proliferative glomerulonephritis (Fig. 4). Intravenous methylprednisolone (500 mg/day for 3 days) was given without improvement in fever and neurologic symptoms and, subsequently, cyclophosphamide (500 mg/day for 1 day) was initiated in conjunction with oral prednisolone 30 mg/day. However, he still complained of confusion and intermittent fever. On the 15th day after admission, plasmapheresis started maintaining oral prednisolone. After 3 sessions in 4 days, the patient improved with no fever and no neurologic abnormality (Fig. 5).
TTP first described by Moschowits in 19241) has overlapping clinical features with SLE on both clinical13) and anatomical12) grounds. Levine and Stearn12) reviewed 151 cases of TTP and found evidence of SLE in 35, although their observations were based solely on postmortem pathologic findings that cannot be considered diagnostic. In the revew or 271 cases of TTP by Amorosi and Ultmann13), 7 patients had positive LE preps and 13 had clinical findings suggestive of SLE. The preliminary criteria for the classification of SLE were not developed at the time of publication. Cecere6) reported a young woman with systemic lupus who developed TTP as a terminal event. In this patient, elevated levels of immune complexes were associated with periods of active lupus but were not detectable at the time she developed TTP. In later series of Ridolfi and Bell of 250 patients with thrombotic thrombocytopenic purpura, only three met the presently accepted criteria for SLE2). Finally, Rothfield14) reported that, in a series of 433 patients with SLE, 2 patients developed TTP.
Although the association of TTP in patients with SLE is frequently mentioned, the pathogenesis of TTP in these patients is not well understood. TTP has similar clinical features with SLE. Hemolytic anemia, thrombocytopenia, fever, central nervous system manifestation and renal disease are common findings not only in TTP but in SLE. However, the blood smear in patients with SLE shows no fragmented red cells except in some cases of lupus related vasculitis. Skin biopsy in SLE shows inflammation around blood vessels, which is typically absent in TTP. The hemolytic anemia is generally CoombsŌĆ▓ test positive in SLE but negative in TTP17). In TTP, immunologic markers (antibodies to DNA, complement, ENA) are negative, although non-SLE associated positive antinuclear antibodies have been found in some patients18). The characteristic pathologic feature of TTP is the hyaline thrombi that occlude capillaries and precapillary arterioles demonstrated in the pancreas, adrenal glands, heart, brain, bone marrow and kidneys which are rarely found in SLE1).
To establish a pathogenic relation between TTP and SLE, various hypotheses have been suggested. However, the precise mechanism is still to be defined. Patients with SLE may present hypercoagulability and be at a greater risk of thrombosis. The possible etiologic factors are immunologic injury of endothelial cells, production of antiendothelial antibody or platelet aggregating factors and, in some cases, the presence of anticardiolipin antibody or lupus anticoagulant3). On the other hand, many immunologic disorders have antedated the development of TTP, including rheumatoid arthritis, ankylosing spondylitis, Sj├ČgrenŌĆÖs syndrome, polymyositis, polyarthritis, GravesŌĆÖ disease and immune thrombocytopenic purpura2).
In our case, on the first admission, the manifestation was compatible with TTP. Classic pentad (microangiopathic hemolytic anemia, thrombocytopenia, neurologic manifestation, aphasia, renal disease, fever) was observed simultaneously and bone marrow biopsy revealed hyaline thrombi occluding capillaries which is a characteristic pathologic feature of TTP. After plasmapheresis, he fully recovered from the illness. Two years later, he was readmitted. At that time, among the 1982 American Rheumatism Association (ARA) criteria for the classification of SLE, he showed renal disorder (proteinuria), neurologic disorder (seizure), hematologic disorders (hemolytic anemia and leukopenia), immunologic disorders (positive anti-ds-DNA and anti-Sm antibody) and antinuclear antibodies. Furthermore, a biopsy of the kidney demostrated lupus nephritis. He was treated with high dose steroid therapy, cyotoxic agent (cyclophosphamide) and, finally, with plasmapheresis and recovered.
Chronologically, TTP can present in patients who were previously diagnosed to have SLE. However, there was no clinical and laboratory evidence of SLE in our patient when he presented with TTP. After 2 years of complete remission, the patient developed a typical case of CNS lupus without evidence of TTP.
In early reports, there were only three patients in whom the diagnosis of thrombotic thrombocytopenic purpura preceded the development of SLE15ŌĆō16). However, clinical information and serologic data are insufficient for the ARA 1982 revised criteria for the diagnosis of SLE in those patients. Their evidence of SLE was the pathologic finding (Libman-Sacks endocarditis, glomerulosclerosis, periadventitial splenic fibrosis) which suggested a ŌĆślupus-typeŌĆÖ disease. The strict application of the present criteria places the diagnosis in doubt. In 1990, Jonsson17) reported a patient diagnosed as SLE two weeks after the diagnosis of TTP. However, at the time of first presentation, direct Coombs test, FANA and anti-ds-DNA were positive, so when he was diagnosed as TTP, maybe he was also suffering from SLE. Another case report was from Simeon-Aznar et al. in 199218). They reported a 30-year-old woman admitted with SLE nine years after developing TTP. When she was first diagnosed as TTP, FANA was positive.
In most of the other cases reported, TTP developed while SLE was in active phase. Several reports documented that the level of immune complex was elevated when lupus was active, but immune complex was not detectable at the time the patient developed TTP6). However, in several associated cases of SLE and TTP, TTP developed when SLE was inactive6,8), sugesting that TTP can develop during the subclinical period of lupus. So, it was postulated that vasculitis from SLE triggered TTP, with the other manifestation of SLE appearing later. However, we cannot fully explain the overall pathophysiologic mechanism in our patient.
We believe that our patient differs from others previously reported, not only in the unusual chronolgical sequence of presentation, but also in the difficulty encountered in establishing a correlation between TTP and SLE, given the long period of time in which the partient was asymptomatic.
Fig.┬Ā1.
Peripheral blood smear. Mild poikilocytosis is seen with some schistocytes and helmet cells. Platelets are markedly decreased in number with some young platelets (Wright stain├Ś1000).
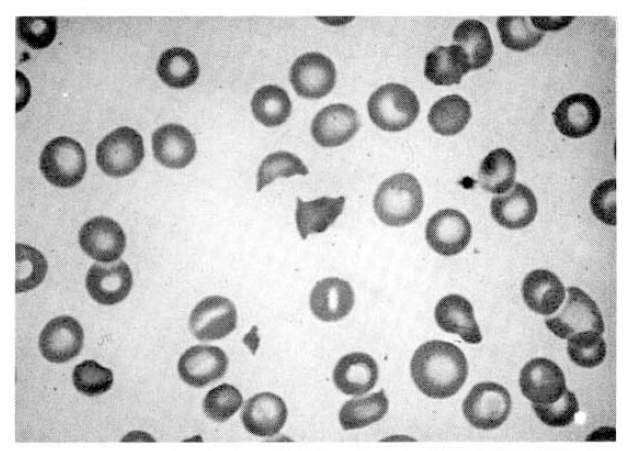
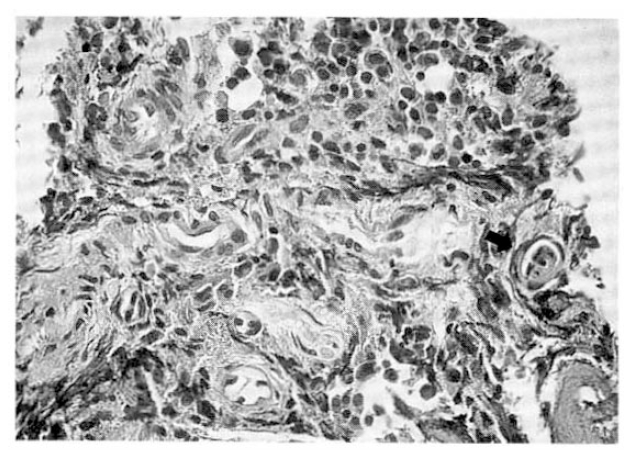
Fig.┬Ā2.
Bone marrow biopsy. Arterioles and venules are prominent and endothelial cell proliferation of vessels is noted with suspicious microthrombi in the lumen (arrow) (HE stain├Ś400).
REFERENCES
1. Moschowitz E. Hyaline thrombosis of the terminal arterioles and capillaries. A hitherto undescribed disease. Proc NY Pathol Soc 1924;24:21.
2. Ridolifi RL, Bell WR. Thrombotic thrombocytopenic purpura: report of 25 cases and review of the literature. Medicine (baltimore) 1981;60:413ŌĆō28.


3. Lim GT, Kim SS, Park SH, Choo WO, Kang DH, Park IS, Chang YS, Yoon Ys, Bang BK. Thrombotic thrombocytopenic purpura-like syndrome associated with systemic lupus erythematosus. Combined treatment with plasmapheresis and fresh frozen plasma infusion. J Korean Med Sci 1992;7(1):66ŌĆō70.



4. Ramkissoon RA. Thombotic thrombocytopenic purpura and systemic lupus erythematosus. California Medicine 1966;104(3):212ŌĆō4.


5. Dekker A, OŌĆÖBrien ME, Cammarata RJ. The association of thrombotic thrombocytopenic purpura with systemic lupus erythematosus: A report of two cases with successful treatment of one. Am J of Med Sci 1974;267(4):243ŌĆō9.

6. Ceccere FA, Yoshinoya S, Pope RM. Fatal thrombotic thrombocytopenic purpura in a patient with systemic lupus erthematosus. Arthritis Rheum 1981;24(3):550ŌĆō553.


7. Finkelstein R, Carter A, Marker A, Rrook JG. Plasma infusions in thrombotic thrombocytopenic purpura complicating systemic lupus erythematosusŌĆöa successful outcome. Postgrad Med J 1982;58:577ŌĆō579.



8. Dixit R, Krieg AM, Atkinson JP. Thrombotic thrombocytopenic purpura developing during pregnancy in a C2-deficient patient with a history of systemic lupus erythematosus. Arthritis Rheum 1985;28(3):341ŌĆō344.


9. Gelfand J, Truong L, Stern L, Pirani CL, Appel GB. Thrombotic thrombocytopenic purpura syndrome in systemic lupus erythematosus. Treatment with plasma infusion. Am J Kidney Dis 1985;6(3):154ŌĆō160.


10. Fox DA, Faix JD, Coblyn J, Fraser P, Smith B, Eeinblatt ME. Thrombotic thrombocytopenic purpura and systemic lupus erythematosus. Ann Rheum Dis 1986;45(4):319ŌĆō322.



11. Itoh Y, Sekine H, hosono O, Tadeuche T, Koide J, Takano Ma, Abe T. Thrombotic thrombocytopenic purpura in two patients with systemic lupus erythematosus. Clinical significance of anti-platelet anti-bodies. Clin Immunol Immunopathol 1990;57(1):125ŌĆō36.


12. Levine S, Dtearn MA. Thrombotic thrombocytopenic purpura and systemic lupus erythematosus. Arch Intern Med 1964;113:826ŌĆō36.


13. Amorosi EL, Ultmann JE. Thrombotic thrombocytopenic purpura: report of 16 cases and review of the literature. Medicine (Baltimore) 1966;45:139ŌĆō159.

14. Rothfield NJ. Systemic lupus erythematosus: clinical and laboratory aspects, Arthritis and Allied Conditions. In: McCarty DJ, ed. Philadelphia: Lea & Febiger, 1979;706.
15. Beigelman PM. Variants of the platelet thrombosis syndrome and their relationship to disseminated lupus. Arch Parthol 1951;51:213ŌĆō23.
16. Siegel BM, Friedman LA, Kressler S, Schwartz SO. Thrombotic thrombocytopenic purpura and lupus erythematosus. Ann Intern Med 1957;47:1022ŌĆō9.


17. Jonsson OG, Fink CW. Systemic lupus erythematosus presenting Thrombotic thrombocytopenic purpura. J Rheumatol 1990;17(7):993ŌĆō4.
-
METRICS

- Related articles
-
A New Onset of Systemic Lupus Erythematosus Developed After Bee Venom Therapy2009 September;24(3)




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


