 |
 |
| Korean J Intern Med > Volume 4(1); 1989 > Article |
|
Abstract
Human genomic DNA samples from 19 Korean patients and 31 controls of known serological DR antigen specificity were studied for insulin-dependent diabetes mellitus (IDDM)-associated variation in HLA-DR╬▓ and - DQ╬▓ restriction fragment length polymorphisms (RFLPs). Genotyping allowed for accurate assignment of HLA-DR types. For HLA-DRw6, a 12kb/DR╬▓/Taq I fragment was decreased in Korean IDDM (p<0.05). However, we could not find an increased frequency of a 12kb/DQ╬▓/Bam HI fragment or decreased frequency of a 3.7kb/DQ╬▓/Bam HI fragment in Korean IDDM. These results suggest a possible protective role of the HLA-DRw6 specificity in IDDM, irrespective of ethnic background, the absence of a specific DQ╬▓ RFLP pattern associated with IDDM in Koreans, and the difference of the Korean population in the genetic of IDDM, compared to the Caucasoid population.
Many genetic studies of different ethnic groups have conclusively shown that the genetic susceptibility to insulin-dependent diabetes mellitus (IDDM) is HLA-linked1ŌĆō5). IDDM is strongly associated with HLA antigen DR4 in Koreans, with DR4 and DRw9 in Japanese, with DR3 and DRw9 in Chinese and with DR3 and DR4 in Caucasoids, while DR2 is unequivocally decreased in IDDM of all populations. However, these different population associations may suggest that it is not the DR antigens themselves, but rather some linked locus that encodes the primary disease-promoting genes6). Furthermore, molecular genetic studies have shown particular DQ╬▓ restriction fragments to be more strongly associated with both susceptibility to, and protection against IDDM7ŌĆō17)
Linkage disequilibrium relationships between class I and class II HLA loci are known to differ markedly in different populations18). Similarly, different linkage relationships between class II genes to occur in different populations, although some of these differences are only evident at the DNA level19ŌĆō21). Moreover, recent studies have provided evidence for some heterogeneity between Asian and Caucasian patients with IDDM, in restriction fragment length polymorphisms (RFLPs) of HLA-DQ as well as - DR17,22,23). Therefore, ethnic comparisons of RFLPs of HLA-DR and - DQ in IDDM might be extremely valuable in identifying specific susceptibility genes or resistance genes, in that the different linkage disequilibrium relationship between class II genes could permit identification of common susceptibility determinants or resistance determinants.
In the present study, we investigated possible differences in the class II HLA DNA polymorphism between HLA-matched Korean IDDM and controls using probes corresponding to the DR╬▓ and DQ╬▓ chain genes. The results are compared with those obtained in insulin-dependent diabetic patients and healthy subjects of other ethnic origin.
Nineteen unrelated patients with IDDM and 31 control subjects, living in Seoul, were selected for study. Clinical criteria for selection of patients included onset before 40 years; body mass index less than 25; ketosis-prone disease and insulin dependence to control symptoms and to prevent basal ketosis. None of the patients had other clinically detectable endocrinopathies or autoimmune disorders. The control subjects were healthy individuals with no personal or family history of diabetes. The patients and controls were selected to match the frequency of DR4 serotype and no further statistical considerations were given.
All subjects were serologically DR-typed and studied by RFLP analysis using class II HLA gene probes. DR-serotyping was by a standard microlym-phocytotoxicity method using antisera supplied for the Third Asia-Oceania Histocompatibility Workshop Conferences in 1986, as previously described3). DR antigens 1,2,3,4,5,w6,7,w8,w9 and w10 were determined in all subjects tested.
For the RFLP studies, genomic DNA was isolated from peripheral blood using standard methods24). Approximately 10ug of DNA was digested with Taq I (40 units, BioLabs) at 65┬░C for 2 hours and Bam HI (70 units, Boehringer) at 37┬░C overnight, under conditions recommended by the manufacturers. Fragments were separated by horizontal electrophoresis through 0.8% agarose gels for 6 hours in TAE-buffer (0.04 M Tris-acetate; 0.001 M EDTA). Southern transfer of separated DNA fragments onto a nylon filter (Amersham) was performed by the alkaline method of Reed and Mann26). cDNA probes for DR╬▓ and DQ╬▓ were used in hybridization27). Probes were labelled by the method of Rigby et al using nick translation kits (Boehringer)28). Prehybridization, hybridization and autoradiography were carried out as previously described by Kohonen-Corish and Serjeantson21).
HLA-DR serotypes were confirmed according to Taq I RFLP patterns as previously described in Caucasoid, Pacific and Chinese populations20,21,29). Figure 1 shows characteristic RFLPs generated by Taq I in conjunction with the cDNA probe HLA-DR╬▓ in Caucasoid populations20). The characteristic banding patterns for different DR specificities show unique RFLPs for all DR antigenic types with the exception of DR3 and DRw6. Approximate fragment sizes of the major bands detected in Caucasoids are summarized in Table 1. HLA-DR3 and DRw6 show two identical RFLP patterns and cannot be distinguished from each other. For the assignment of HLA-DR3 and DRw6, the Taq I fragments hybridizing with the DQ╬▓ probe were used (Fig. 2). All samples of DR3 specificity had 4.6 and 3.5 kb fragments. In addition to these two fragments, a 5.5 kb or 3.0 kb fragment was associated with DRw6. Where occasional discrepancies occurred between serological and DNA-DR assignment, the serology and the RFLP studies were re-examined in the Canberra tissue typing laboratory of Australian National University.
All RELP difference between IDDM patients and control within each of the DR specificities were tested by chi-square tests or, where appropriate, by FisherŌĆÖs exact test from 2├Ś2 contingency tables.
There were occasional discrepancies between serotypes assigned in the Seoul Laboratory and genotypes assigned in the Canberra Laboratory. These discrepancies arose from inaccurate assignment of some DR3, DR4, DR5 and DRW6 positive samples or from failure to detect those specificities (Table 2), Where discrepancies occurred between serological and DNA-DR assignments, the serology was re-examined and in all cases the DNA-DR typing were shown to be correct.
DR3 showed only a large variant (12 kb) whereas two DNA subtypes, a large variant and small variant (10 kb) has been found in Caucasoids. There were two patterns indentified in DR5 specificity (Fig 3). One pattern was similar to that observed in Caucasoids, which had 12, 6.0, and 4.2 kb fragments. The other was a subtype of DR5 with 10 and 4.4 kb fragments, DR5* NAURU, which was first reported in Micronesians from Nauru. Also, DRw6 showed two DNA subtypes characterized by large (12 kb) or small (10 kb) variants that were evident in other ethnic groups (Fig 4). The frequency of the 12 kb/DR╬▓/Taq I fragment in the patients with IDDM was significantly lower than that in the controls (p<0.05). All of these results were summarized in Table 3.
With the DR2 specificity, three RFLP subtypes could be defined using the Taq I fragments hybridized with the DQ╬▓ probes (Table 4). These subtypes were identical with known cellular HLA-D specificities, such as Dw2, Dw12 and Dw ŌĆśAZHŌĆÖ (Table 4 & Fig 5). These subtypes did not demonstrate significant differences in the frequencies between the patients and the controls.
The Bam HI RFLPs hybridizing with the DQ╬▓ probe are shown in Figure 6 and the frequencies of DNA fragments are summarized in Table 5. Decrease of the 3.7 kb fragment and increase of the allelic 12 kb fragment in DR4 postive patients as well as in all IDDM patients were not confirmed, in contrast to those reported in Caucasoids. Furthermore, the 12 kb fragment was predominant not only in IDDM patients but in healthy DR4 positive individuals.
Taq I site polymorphisms of the HLA-DR genes are clearly associated with HLA-DR serological specificities in Koreans as previously reported in other ethnic groups20,21,29). Our study shows unique RFLP patterns associated with DR1 to DRw10, with the exception of DR3 and DRw6. However, with the DQ╬▓ probe, a clear difference is seen between DR3 and DRw6. In the past, the serotyping for accurate assignment of some HLA-DR specificities, such as DR3, DR4, DR5 and DRw6, has occasionally been difficult due to the lack of monospecific typing sera and due to cross-reaction between the DR specificities (2, 23). Recently, cellular and molecular genetic studies have been several subtypes within each of the DR serotypes, such as DR2, DR3, DR4, DR5 and DRw612,13,20,21,30,31). A restriction endonuclease, Taq I, has consistently proved to be one of the most informative in detecting RFLPs in man and use of Taq I has clear advantages in examining DR╬▓ RFLPs20). Therefore, genotyping with restriction endonuclease Taq I and cDNA probes of DR╬▓ and DQ╬▓ for accurate assignments of the DR specificities is necessary to evaluate HLA and disease associations and to carry out the ethnic comparisons in HLA-associated diseases.
We have previously suggested the possible protective role of DRw6 in Korean IDDM patients using serotyping of HLA-DR antigens3). Interestingly, we also found a decreased frequency of the 12 kb/DR╬▓/Taq I fragment within the DRw6 specificity in the present study. Genotyping allows for accurate assignments of the DRw6 specificity, which in the past has been difficult due to the lack of monospecific typing sera. Indeed, in the definitive study of HLA and IDDM in the Eighth International Histocompatibility Workshop, tabulations of HLA DRw6 was omitted due to technical difficulties32). Thus, the possible protective role of DRw6 in IDDM may have been previously overlooked23). DR2 and DRw6 show similarities in their DQ╬▓ and DQ╬▒ RFLPs, with different combinations of these occuring in different DR2 and DRw6 positive cells in Koreans as well as Caucasoids21,33). The 3.0 kb/DQ╬▓/Taq I fragment that is associated with the Dw2 specificity is also found within DRw6 specificity, and the frequency of subtype of Dw6 having this fragment is significantly decreased in the Caucasoid and North Indian IDDM patients23,34). However, 12 kb/DR╬▓/Taq I subtypes of DRw6 were predominant in Koreans and were associated with the 5.5kb/DQ╬▓/Taq I fragment33). Thus, further RFLP studies of genomic DNA on the protective role of DRw6 in Korean IDDM will be needed.
Within the DR2 specificity, three RFLP patterns subtypes could be defined using DQ╬▓ probes in the Korean population. These different DQ╬▓ RFLP patterns associated with DR2 are known to correspond with cellular HLA-D specificities, such as Dw2, Dw12 and Dw ŌĆśAZHŌĆÖ12). In DR2 positive Caucasian individuals, a decrease in the frequencies of Dw2 and Dw12 was observed. However, the majority of DR2 positive Caucasian patients had the Dw ŌĆśAZHŌĆÖ (or LD-MN2) specificity, suggesting that this haplotype conferred susceptibility to IDDM12, 35). Thus, the negative association of DR2 with Korean IDDM should be reevaluated further at the DNA level, to determine whether Dw ŌĆśAZHŌĆÖ in Koreans is also conferring susceptibility to IDDM.
In our study, neither the 12 kb/DQ╬▓/Bam HI fragment was preferentially increased, nor the 3.7 kb/DQ╬▓/Bam HI fragment decreased, in DR4 positive Korean patients and in all patients, in contrast to the results in the Caucasoid and North Indian patients7,8,10,11,15,23,31,34). These results were similar to those in Canton Chinese and Japanese, and the 12 kb subtype occurred in most healthy DR4 positive Korean and Chinese populations where IDDM was rare7,23). Furthermore, the 12 kb fragment was associated with Bw62 and DR4 in both Caucasoid patients and healthy controls23). Thus, the 12 kb fragment could not be a new disease specific marker for the entire IDDM associated haplotype, but a marker for a subtype of DR4. And, the increase of the 12 kb fragment in Caucasoid patients might be due to the haplotype specific variation that can be commonly detected by RFLPs in the MHC20,23). Therefore, at the present time, no definitive evidence for the HLA-DQ╬▓ systemŌĆÖs contribution to the development of IDDM in Koreans as well as Japanese is present, as in Caucasians17).
Recently, using the analysis of diabetic DNA sequences, interesting results were reported. It was observed that the amino acid at position 57 of the DQ╬▓ chain determined IDDM susceptibility16,36). More than 90% of the IDDM patients were homozygous for nonaspartic acid (non-Asp-57), and none were homozygous for aspartic acid (Asp-57) in the Caucasoid population. However, this is different in the case of the Japanese, where more than 24% of the IDDM patients are homozygous for Asp-57 of the DQ╬▓ chain17). Thus, the absence of a specific DQ╬▓ RFLP pattern in this Korean IDDM population might be due to the fact that Korean IDDM occurs in patients with Asp-57 specificities. If this is the case, Oriental IDDM might be of basically a different etiology from that of Caucasians. Interestingly, as assessed by this DQ╬▓ phenotype, the absolute annual risk of disease for genetically susceptible and non-susceptible individuals was calculated as 70/100,000 for individuals who were non-Asp-57 homozygotes, but only 0.65/100,000 for those with at least one Asp-57 allele37). The latter risk estimate approximates the overall annual incidence of IDDM in Korea as well as in Japan. These findings also suggest that the HLA-DQ╬▓ system does not confer susceptibility to Korean IDDM and there is some heterogeneity between Asian and Caucasoid populations, in the etiopathogenesis of IDDM.
In conclusion, this study has provided evidence for some heterogeneity in the genetics of IDDM between Korean and Caucasian populations. So, the population-specific (haplotype-specific) and IDDM-associated variation of the HLA-class II RFLPs, and diabetic DNA sequence of the HLA-DQ╬▓ system in different ethnic groups must be simultaneously examined to localize the region of susceptibility of IDDM and to find out the heterogeneity in the contribution of genetic factors to the development of IDDM.
Acknowledgments
We are most grateful to Dr. S.W. Serjeantson and Dr. M.R.J. Kohonen-Corish, John Curtin School of Medical Research, Australian National University, for providing us with some RFLP data and excellent discussion for the present study, and Dr. B. Mach, University of Geneva, for providing us with the cDNA probes. We also thank Dr. D.I. Kim, Honorary President of the Korean Association of Internal Medicine, for providing us with the research grant.
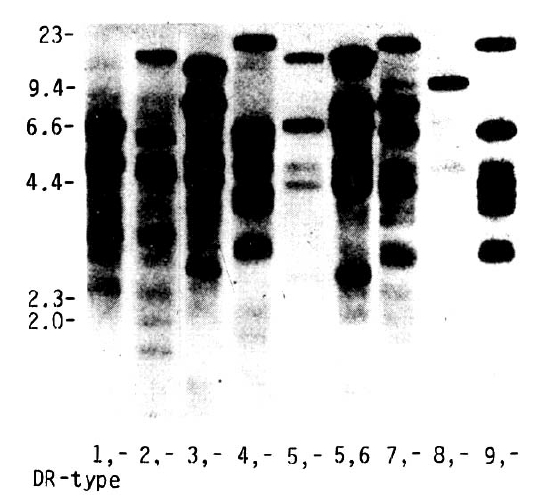
Fig.┬Ā3
HLA-DR╬▓ hybridization of Taq I-digested genomic DNA showing the different RFLP patterns detected within DR5 specificity (1: DNA-DR5*NAURU, 2: DNA-DR5).
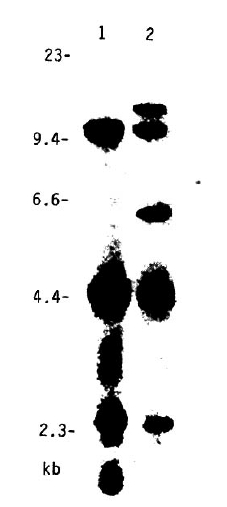
Fig.┬Ā4
HLA-DR╬▓ hybridization of Taq I-digested genomic DNA showing the different RFLP patterns detected within DRw6 specificity (1: DNA-DRw6 small, 2: DNA-DRw6, large).
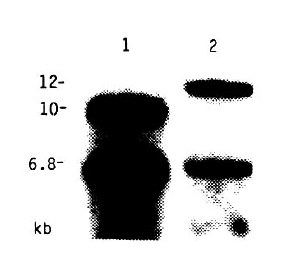
Fig.┬Ā5
HLA-DQ╬▓ hybridization of Taq I-digested genomic DNA showing the different RFLP patterns detected within DR2 specificity (1: HLA-Dw2, 2: HLA-Dw12, 3: HLA-Dw ŌĆśAZHŌĆÖ).

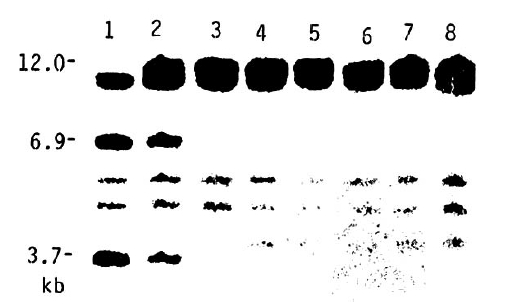
Fig.┬Ā6
HLA-DQ╬▓ hybridization of Taq I-digested genomic DNA showing the different RFLP patterns in HLA-DR4 positive controls (1ŌĆō4) and patients (5ŌĆō8).
Table┬Ā1
Approximate Fragment Sizes of the Major Bands Detected with the HLA-DR╬▓ Probe after Digestion with Taq I, within Different DR Specificities in Cells of Caucasoid Origin
Table┬Ā2
HLA-DR Antigen Frequencies (%) in Individuals (n = 50) studied by HLA-DR Serotyping and Genotyping
| HLA antigens | Serotyping | Genotyping |
|---|---|---|
| DR3 | 8.0 | 4.0 |
| DR4 | 52.0 | 42.0 |
| DR5 | 4.0 | 32.0 |
| DRw6 | 0.0 | 26.0 |
Table┬Ā3
DNA-DR Antigen Frequencies (%) in IDDM Patients and Controls
| DNA-DR | IDDM (n = 19) | Controls (n = 31) |
|---|---|---|
| DR3 | ||
| ŌĆāŌĆāSmall (10 kb) | ŌĆō | ŌĆō |
| ŌĆāŌĆāLarge (12 kb) | 5.3 | 3.2 |
| DR5 | ||
| ŌĆāŌĆāDR5 | 5.3 | 12.9 |
| ŌĆāŌĆāDR5*NAURU | 26.3 | 19.4 |
| DRw6 | ||
| ŌĆāŌĆāSmall (10 kb) | 21.0 | 9.7 |
| ŌĆāŌĆāLarge (12 kb) | 0.0** | 19.4 |
REFERENCES
1. Tiwari JL, Terasaki PI. HLA and Disease Associations. 1st ed. 185. New York: Springer-Verlag, 1985.
2. Huh KB, Lee HC, Park K, Lee SY. HLA distribution in Korean patients with insulin-dependent diabetes mellitus. Yonsei J 27:54. 1986;(English).

3. Rhee BD, Choi SJ, Park SW, Choi DS, Han H, Kim GY, Cho BY, Lee HK, Koh CS, Min HK. HLA and insulin-dependent diabetes mellitus in Koreans. Korean J Intern Med 2:135. 1987;(English).



4. Min HK. A view of present day diabetes in South Korea. In: Krall LP, Alberti KGMM, Turtle JR, eds. World Book of Diabetes in Practice. 3:350. Amsterdam: Elsevier Science Publishers BV, 1988.
5. Bashir H, Juji T, Moffitt P. Diabetes mellitus. In: Simons MJ, Tait BD, eds. Proceedings of the Second Asia and Oceania Histocopmatibility Workshop Conference; p. 332. Toorak: Immunopublishing, 1983.
6. Strominger JL. Biology of the human histocompatibility leukocyte antigen (HLA) system and a hypothesis regarding the generation of autoimmune diseases. J Clin Invest 77:1411. 1966.



7. Owerbach D, Lernmark ├ģ, Platz P, Ryder LP, Rask L, Peterson PA, Ludvigsson J. HLA-D region ╬▓-chain DNA endonuclease fragments differ between HLA-DR identical healthy and insulin-dependent diabetic individuals. Nature 303:815. 1983.


8. Owerbach D, H├żggl├Čf B, Lernmark ├ģ, Holmgren G. Susceptibility to insulin-dependent diabetes defined by restriction enzyme polymorphism of HLA-D region DNA. Diabetes 33:958. 1984.


9. Cohen D, Cohen O, Marcadet A, Massart C, Lathrop M, Deschamps I, Hors J, Schuller E, Dausset J. Class II HLA-DC B-chain DNA restriction fragments differentiate among HLA-DR2 individuals in insulin-dependent diabetes and multiple sclerosis. Proc Natl Acad Sci (USA) 81:1774. 1984.



10. Cohen-Haguenauer O, Robbins E, Massart C, Busson M, Deschamps I, Hors J, Lalouel J-M, Dausset J, Cohen D. A systematic study of HLA class II-╬▓ DNA restriction fragments in insulin-dependent diabetes mellitus. Proc Natl Acad Sci (USA) 82:3335. 1985.



11. Nepom BS, Palmer J, Kim SJ, Hansen JA, Holbeck SL, Nepom GT. Specific genomic markers for the HLA-DQ subregion discriminate between DR4* insulin-dependent diabetes mellitus and DR4* seropositive juvenile rheumatioid arthritis. J Exp Med 164:345. 1986.



12. Cohen N, Brautbar C, Font M-P, Daussart J, Cohen D. HLA-DR2 associated Dw subtypes correlated with RFLP clusters: Most DRw IDDM patients belong to one of these clusters. Immunogenetics 23:84. 1986.


13. Festenstein H, Awad J, Hitman GA, Cutbush S, Groves AV, Cassel P, Oilier W, Wachs JA. New HLA DNA polymorphisms associated with autoimmune diseases. Nature 322:64. 1986.


14. B├Čhme J, Carlsson B, Wallin J, M├Čller E, Person B, Peterson PA, Rask L. Only one DQ-╬▓ restriction fragment pattern of each DR specificity is associated with insulin-dependent diabetes. J Immunol 137:941. 1986.

15. Michelsen B, Lermmark ├ģ. Molecular cloning of a polymorphic DNA endonuclease fragment associates insulin-dependent diabetes mellitus with HLA-DQ. J Clin Invest 79:1144. 1987.



16. Todd JA, Bell Jl, McDevitt HO. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature 329:599. 1987.


17. Aparicio JMR, Wakisaka A, Takada A, Matsuura N, Aizawa M. HLA-DQ system and insulin-dependent diabetes mellitus in Japanese: does it contribute to the development of IDDM as it does in Caucasians? Immunogenetics 28:240. 1988.


18. Baur MP, Neugebauer M, Albert ED. Reference tables of two-locus haplotype frequencies for all MHC marker loci. In: Albert ED, Baur MP, Mayr WR, eds. Histocompatibility Testing 1984. 677. Berlin: Springer-Verlag, 1984.

19. Oudshoorn M, Schreuder GMT, Campbell EM, du Toit ED. Segregation of DQ and DR: ŌĆ£Exceptions to the ruleŌĆØ. In: Albert ED, Baur MP, Mayr WR, eds. Histocompatibility Testing 1984. 419. Berlin: Springer-Verlag, 1984.

20. Kohonen-Corish MRJ, Serjeantson SW. HLA-DR gene DNA polymorphisms revealed by Taql correlate with HLA-DR specificities. Hum Immunol 15:263. 1986.


21. Kohonen-Corish MRJ, Serjeantson SW. RFLP analysis of HLA-DR and-DQ genes and their linkage relationships in the Pacific. Am J Hum Genet 39:751. 1986.


22. Hitman GA, Sachs J, Casell P, Awad J, Bottazzo GF, Tarn AC, Schwartz G, Monson JP, Festenstein H. A DR3-related DX╬▒ gene polymorphism strongly associates with insulin-dependent diabetes mellitus. Immunogenetics 23:47. 1986.


23. Kohonen-Corish MRJ, Serjeantson SW, Lee HK, Zimmet P. Insulin dependent diabetes mellitus: HLA-DR and -DQ genotyping in three ethnic groups. Disease Markers 5:153. 1987.

24. Manlatis T, Fritsch EF, Sambrook J. Molecular Cloning A Laboratory Manual. New York: Cold Spring Harbor Laboratory, 1982.
25. Southern EM. Detection of specific sequences of DNA fragments separated by gel electrophoresis. J Mol Biol 98:503. 1975.


26. Reed KC, Mann PA. Rapid transfer of DNA from agarose gels to membranes. Nucleic Acid Res 13:7207. 1985.



27. Long EO, Wake CT, Strubin M, Gross N, Accolla RS, Carrell S, Mach B. Isolation of distinctive cDNA clones encoding HLA-DR╬▓ chains by use of an expression assay. Proc Natl Acad Sci (USA) 79:7465. 1982.



28. Rigby PWJ, Dieckmann M, Rhodes C, Berg P. Labeling deoxyribonucleic acid to a high specific activity in vitro by nick translation with DNA polymerase 1. J Mol Biol 113:237. 1977.


29. Kohonen-Corish MRJ, White BS, Hawkins BR, Serjeantson SW. HLA-DR and DQ RFLPs in Hong Kong Chinese. In: Aizawa M, Natori T, Wakisaka A, Konoeda Y, eds. HLA in Asia-Oceania 1986. 454. Sapporo: Hokkaido University Press, 1986.
30. Segall M, Noreen H, Schluender L, Bach FH. DNA restriction fragment length polymorphisms characteristic for Dw subtypes of DR2. Immunol 15:336. 1986.

31. Kim SJ, Holbeck SL, Nisperos B, Hansen JA, Maeda H, Nepom GT. Identification of a polymorphic variant associated with HLA-DQw3 and characterized by specific restriction sites within the DQ╬▓-chain gene. Proc Natl Acad Sci (USA) 82:8139. 1985.



32. Svejgaard A, Platz P, Ryder LP. Insulin-dependent diabetes mellitus. In: Terasaki PI, ed. Histocompatibility Testing 1980. 638. Los Angeles: UCLA, Tissue Typing Laboratory, 1980.
33. Kohonen-Corish MRJ, Dunckley H, Serjeantson SW. HLA-DR and -DQ DNA genotyping in seven populations of Asia-Oceania and Australia. Tissue Antigens 32:32. 1988.


34. Fletcher J, Odugbesan O, Mijovic C, Mackay E, Bradwell AR, Barnett AH. Class II HLA DNA polymorphisms in type 1 (insulin-dependent) diabetic patients of North Indian origin. Diabetologia 31:343. 1988.


35. Bach FH, Rich SS, Barbosa J, Segall M. Insulin-dependent diabetes associated HLA-D region endcode determinants. Hum Immunol 12:59. 1985.


36. Morel PA, Dorman JS, Todd JA, McDevitt HO, Trucco M. Aspartic acid at position 57 of the HLA-DQ╬▓ chain protects against type 1 diabetes: A family study. Proc Natl Acad Sci (USA) 85:8111. 1988.



37. Dorman JS, Trucco M. Determining the absolute risk of developing insulin-dependent diabetes mellitus (IDDM) by HLA-DQ phenotype: A new interface between epidemiology and molecular biology. In : Satellite Congress of the 13th IDF Congress ŌĆ£Epidemiology of DiabetesŌĆØ; 1988. (Abstract).
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 16,422 View
- 35 Download
- Related articles
-
Polymorphism of Glucokinase Gene in Non-Insulin Dependent Diabetes Mellitus1994 January;9(1)
HLA and Insulin-Dependent Diabetes Mellitus in Koreans1987 July;2(2)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


