 |
 |
| Korean J Intern Med > Volume 4(1); 1989 > Article |
|
Abstract
Thirty-nine patients with chronic HBV infection and 38 normal persons were investigated by simultaneous assay of T suppressor cell function and enumeration of T-lymphocyte subsets by monoclonal antibodies. In patients with chronic active hepatitis B (CAH-B), T suppressor cell activity (17.8┬▒8.8%) was significantly lower than in healthy HBsAg carriers (35.4 ┬▒ 12.3%) and normal control persons (38.3┬▒16.3%). The proportions of T-lymphocyte subsets in patients with CAH-B were not different from those of healthy HBsAg carriers and control persons. No correlation was observed in between percentage suppression and proportions of T-lymphocyte subsets. These findings suggest that in the absence of a simultaneous assay of function, enumeration of T-lymphocyte subsets by using monoclonal antibodies is an inadequate assessment of immune regulation.
Chronic active hepatitis B (CAH-B) is a progressive inflammatory liver disease caused by chronic hepatitis B virus (HBV) infection. Since HBV is not directly cytopathic to infected hepatocytes1), it is generally accepted that liver cell injury observed in chronic HBV infection may be dependent on the host-determined immune responses directed at viral and self-antigens expressed on the surface of infected hepatocytes2,3). These immunological responses may be regulated by T-lymphocyte subsets, especially T helper and T suppressor cells. Several authors have indeed observed differences in T-lymphocyte subsets among patients with CAH-B4,5). Alterations in numerical balance between T helper and T suppressor cells, as determined by monoclonal antibodies, are often extrapolated to the functional defects of immunoregulatory T-lymphocytes6). On the basis of these observatons there has been an emerging concept that the disturbance of immunoregulatory T-lymphocyte number or function may be a primary pathogenetic determinant in ongoing liver cell injury in patients with CAH-B7,8). However, since this concept is not always justified by other workers, in the light of T suppressor cell number9ŌĆō11) as well as the relation between T suppressor cell number and function12), it is still of uncertain significance for the understanding of the pathogenetic mechanism of CAH-B.
In the present study, we assessed the relationship between T-lymphocyte subsets, defined by monoclonal antibodies, and T suppressor cell function in patients with CAH-B and healthy HBsAg carriers. T suppressor cell function was simultaneously evaluated with the enumeration of T-lymphocyte subsets in each case.
Seventy-seven subjects entered into this study. The study population consisted of 19 healthy HBsAg carriers (group I) with normal serum aminotransferases during an observation period of at least 6 months and 20 patients with HBsAg-positive CAH (group II). In all patients with CAH-B, the histological diagnosis was assessed according to the suggestions of an International Committee (Forgarty International Center Proceedings, 1976)13). Thirty-eight normal persons (group III) without clinical evidence of liver diseases and HBV infection were included as the control group. Table 1 summarizes some clinical and laboratory data of our cases. No patients were on immunosuppressive therapy before the present study. Patients with alcoholic liver diseases, advanced liver cirrhosis, primary hepatocellular carcinoma, organ transplantation, and known lymphoproliferative diseases were excluded from this study. Serological studies of HBs-Ag and anti-HBs were performed by enzyme immunoassay (EIA), and of anti-HBc, HBeAg and anti-HBe with radioimmunoassay (RIA) using commercially available reagent kits (Ausria II).
PBMNC were isolated from heparinized blood through Ficoll-Hypaque density gradient centrifugation14). Blood samples from each subject were collected by venipuncture into a heparinized (10 U/ml of blood) syringe. PBMNC were separated by centrifugation on a Ficoll-Hypaque gradient, and washed three times with phosphate-buffered saline (PBS, 0.15M, pH 7.2) without calcium and magnesium. The viable cells were counted by using 0.16% trypan blue (Gibco) in physiological solution and resuspended at a cell density of 106 cells/ml in RPMI 1640 medium (Flow) supplemented with 5% fetal bovine serum (FBS, Gibco). Generally about 95% viability and 87 to 90% lymphocytes were obtained.
T-lymphocyte subsets were determined by indirect immunofluorescent staining using mouse antihuman T-lymphocyte monoclonal antibodies (Ortho Pharmaceutical Corp., Raritan, NJ, USA). Three monoclonal antibodies were used; OKT 3 reacting with all peripheral blood T cells, OKT 4 directed to T helper cells, and OKT 8 identifying a T-lymphocyte subset with both suppressor and cytotoxic functions15, 16). A volume of 0.1ml of PBMNC suspension (2 ├Ś 106/ml) was mixed with 5 ul of each reconstituted monoclonal antibody (50 ug/ml) and incubated at 4┬░C for 30 min. After incubation, the cells were washed three times in cold PBS and resuspended in 0.1 ml of PBS. They were stained at 4┬░C for 30 min with fluorescein-conjugated goat antimouse IgG (GAM/lgG-Fc/FITC, Nordic Immunological Lab., BV., Nordic Pharmaceuticals, The Netherlands) and then washed three times in cold PBS and resuspended in a drop of PBS. Membrane immunofluorescence was evaluted with an AO immunofluorescent microscope equipped with an exciting filter, a barrier filter, and incident illumination. Three to four hundred cells were counted for the calculation of percentage of positively stained cells.
The assay for inducible suppressor cells was carried out according to the method described by Sakane and Green17) with minor modifications. The PBMNC isolated from heparinized venous blood through Ficoll-Hypaque density gradient centrifugation were adjusted to a concentration of 5 ├Ś 106 cells per 2 ml RPMI 1640 medium and incubated with or without ConA (Concanavalin A, Sigma, 10 ug/ml) for 48 hours at 37┬░C under 95% air/5% CO2. After this incubation, ConA-activated cells were treated with mytomycin C (Sigma, 50ug/ml) for 30 min and extensively washed with complete medium. Next, the cells were washed three times with 0.3M methyl-╬▒-D-mannopyranoside (Sigma) in PBS to remove ConA, washed again, and resuspended in complete medium at a concentration of 1 ├Ś 106 cells per ml. Control cells received no ConA on culture inititation but were cultured alone for 48 hours, pulsed with ConA at harvest, and washed with methyl-╬▒-D-mannopyranoside in the same manner as the ConA-generated cells. In determining the effect of suppressor cells on the allogeneic mixed lymphocyte reaction18), 0.5 ├Ś 105 responder mononuclear cells were cocultured with 0.5 ├Ś 105 control or ConA-generated cells, which are autologous to the responder cells in the presence of 0.5 ├Ś 105 mytomycin C-treated allogeneic stimulator cells. Quadriplicate cultures for each point were incubated for 4 days at 37┬░C in 95% air/5% CO2 at 100% humidity. Eighteen hours before harvest, 1 uCi/well of [3H] thymidine (Tritiated thymidine, SA: 20 Ci/mM, The Radiochemical Centre, Amersham) was added to each well. The cells were then collected from the wells onto a fiberglass filter by use of a cell harvest device (Cell harvester 530, Flow). The cell pellets on the filter paper were dissolved for 4 hours at room temperature in 2 ml of Instagel (Packard). The radioactivity was counted by a Packard Liquid Scintillation Spectrometer (Model 4530). Percent suppression was determined by the formula: % suppression = [1-cpm (S)/cmp (C)] ├Ś 100, where cpm (S) is the net counts per minute in culture to which ConA-activated suppressor cells had been added, and cpm (C) is the net counts per minute in cultures to which control cells had been added.
The statistical analysis of the data was done with multiple regression analysis and the StudentŌĆÖs t-test.
The determination of T-lymphocyte subsets using OK monoclonal antibodies was carried out simultaneously with the assay for suppressor cell function in each case. As shown in Table 2, the numerical distribution of T-lymphocyte subset in patients with HBsAg-positive CAH was not significantly different from those of healthy HBsAg carriers and control persons.
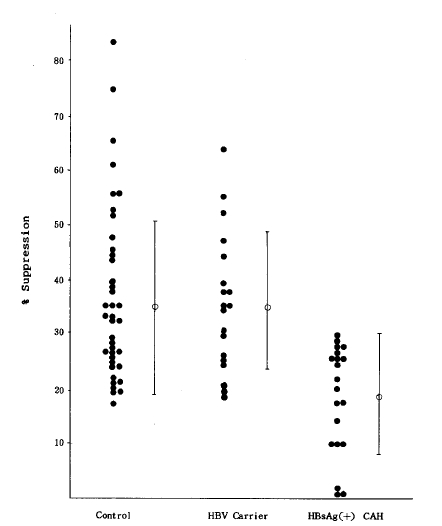
ConA-induced suppressor cell activity in the study groups is summarized in Table 2 and depicted in Figure 1. In patients with HBsAg-positive CAH, the mean level of suppressor cell activity (17.8 ┬▒ 8.8%) was significantly (p<0.005) lower than that of control persons (38.3 ┬▒ 16.3%) and healthy HBsAg carriers (35.4 ┬▒ 12.3%). There was, however, no statistical difference in value between healthy HBsAg carriers and control persons. The presence of HBeAg in group II patients did not influence the suppressor cell activity.
As shown in Table 3, no correlation was observed between percent suppression and the proportion of T3+, T4+ and T8+ cells and the T4/T8 ratio in patients with CAH-B as well as in healthy HBsAg carriers.
The cardinal histologic feature of CAH-B is the presence in the periportal tissue of piecemeal necrosis with or without spotty necrosis19). This type of liver cell necrosis is mediated by hepatocyte-lymphocyte interaction with subsequent hepatocellular degeneration. In recent years, there have been a considerable advance in the understanding of both the viral antigen display on infected hepatocytes and resultant host immune responses. By using monoclonal antibodies to HBV antigens, HBcAg has been identified as the major viral product expressed on the surface of liver cells isolated from patients with CAH-B20). In these patients, moreover, the composition of inflammatory infiltrates in areas of piecemeal necrosis as well as spotty necrosis has been found to consist largely of T cytotoxic cells21, 22). All these findings indicate that, whatever the target antigen (s) for T cytotoxic cells infiltrated in the areas of liver cell necrosis may be, the normal state of tolerance to hepatocytes may be compromised during chronic HBV infection, resulting in the emergence of autoreactive hepatotoxic effector lymphocytes with concomitant hepatocellular injury23). The importance of a balance between T helper and T suppressor cells in maintaining the normal status of tolerance has recently been illustrated and abnormalities in these immunoregulatory T-lymphocyte subsets have indeed been associated with a number of human diseases24). Since the availability of monoclonal antibodies that easily identify immunoregulatory T-lymphocyte subsets has stimulated wide interest in the study of immune control mechanisms, the number or proportion of each regulatory T-lymphocyte, as defined by monoclonal antibodies, has also been studied in patients with CAH-B and the findings were often extrapolated to the functional defects in suppressor T cells6, 25).
The current study was undertaken to address two issues of interest related to the immunoregulatory T-lymphocytes in patients with CAH-B; first, whether the functional defects in T suppressor cells are associated with a decrease in T suppressor cell number and second, whether the functional alterations of T suppressor cells in peripheral blood are a causal factor influencing the degree of inflammatory liver damage, or are merely changes secondary to the presence of liver injury. The results of the present study, while confirming a significant defect in T suppressor cell function in patients with CAH-B as compared to healthy HBsAg carriers and control persons, did not show an upset in the numerical distribution of T-lymphocyte subsets in all study groups. Although this finding, a contrasting relation between T suppressor cell number and function, has also been identified in patients with idiopathic glomerulonephritis26) and chronic hepatitis12), the immunological meaning of the data available here in are indeed open to several interpretations. The results of counting T-lymphocyte subsets by means of monoclonal antibodies in patients with CAH-B should be interpreted cautiously, since the correlation between the antigen properties of T-lymphocyte surface and functional classification of these of cells is not yet clearly defined. Another problem of interpretation concerns the fact that OKT 8-positive cells are not a homogenous group, since OKT 8-specific antibody, directed against the receptor for HLA Class I antigens, reacts not only to T suppressor cells but also to T cytotoxic cells. Thus, the results of counting OKT 8-defined cells in peripheral blood may not be an accurate estimate of T suppressor cell number. This impression, in conjunction with several recent reports showing variable results in the enumeration of immunoregulatory T-lymphocyte subsets, provides substantial evidence in favor that OKT 8 monoclonal antibody is not a suitable antibody for an accurate enumeration of T suppressor cells and; therefore, an accurate estimate of immune regulation can, at the present time, be obtained only by functional assays.
Recent results21, 22) observed in immunohistochemical staining of liver tissues taken from patients with CAH-B have shown that hepatocytes in the areas of piecemeal necrosis and spotty necrosis express Class I major histocompatibility complex (MHC) products which are important antigens in MHC-restricted cytotoxic action of T cytotoxic cells27). These observations, together with the finding that inflammatory cells in the areas of piecemeal necrosis as well as spotty necrosis have been found to consist largely of T cytotoxic cells, indicate strong evidence that liver cell necrosis observed in these areas is caused by MHC-restricted cytotoxic action, directed against HBcAg or self-antigen expressed on the surface of infected hepatocytes, of T cytotoxic cells. Since these findings are not observed in patients with chronic persistent hepatits21), it is logical to speculate the possibility that continous liver cell inflammation may depend on the expression on the surface of infected liver cells of HLA Class I and II antigens which are expressed only very weakly, if at all, on the surface of normal human liver cells28). Thus, enhanced expression caused by HBV infection of HLA antigens (Class I and II), together with expression of HBcAg or selfantigen(s), on the surface of infected hepatocytes can continuously stimulate T helper and/or T cytotoxic cells to react against infected hepatocytes, with concomitant hepatocellular degeneration in the liver and reduction of T suppressor cell function in peripheral blood.
In conclusion, although we do not yet know the precise mechanism of liver cell necrosis observed in patients with CAH-B, we speculate that the primary pathogenetic determinant in ongoing liver cell injury is not the defect in T suppressor cell function, but is the simultaneous expression on the surface of infected hepatocytes of HLA antigens and virusdetermined antigen(s) and/or self-antigen(s). These antigens can indeed provoke continuous immune responses to generate MHC-restricted T cytotoxic cells leading to hepatocellular degeneration.
Table┬Ā1.
Clinical and Laboratory Findings in Study Groups
| Group | Number | Sex (M/F) | Age (yr) (mean ┬▒ SD) | SGPT (IU/L) (mean ┬▒ SD) |
|---|---|---|---|---|
| Healthy HBsAg carriers(1) (Group I) | 19 | 11/8 | 40.0 ┬▒ 10.0 | 25.4 ┬▒ 8.5 |
| HBsAg-positive CAH(2) (Group II) | 20 | 14/6 | 34.4 ┬▒ 8.4 | 227.2 ┬▒ 180.8 |
| Control persons(3) (Group III) | 38 | 15/23 | 41.5 ┬▒ 1 2.0 | 16.6 ┬▒ 7.3 |
Table┬Ā2.
Mean Percentage Suppression, Total T-lymphocytes (T3 +), T helper Lymphocytes (T4 +). Suppressor/Cytotoxic Cells (T8 +), and Helper/Supressor Ratio (T4/T8) in Study Groups
|
Mean ┬▒ SD
|
|||||
|---|---|---|---|---|---|
| % Suppression | % T3 + | % T4 + | % T8 + | ratio T4/T8 | |
| Healthy HBsAg carriers (Group I, n = 19) | 35.4 ┬▒ 12.3(a) | 66.8 ┬▒ 5.2 | 39.7 ┬▒ 9.1 | 23.7 ┬▒ 4.5 | 1.75 ┬▒ 0.5 |
| HBsAg-positive CAH (Group II, n = 20) | 17.8 ┬▒ 8.8(b) | 65.4 ┬▒ 6.2 | 42.0 ┬▒ 5.2 | 24.2 ┬▒ 3.7 | 1.8 ┬▒ 0.3 |
| Control persons (Group III, n = 38) | 38.3 ┬▒ 16.3(c) | 70.0 ┬▒ 7.3 | 44.3 ┬▒ 8.8 | 23.0 ┬▒ 3 8 | 1.9 ┬▒ 0.4 |
Table┬Ā3.
Correlation Between Percentage Suppression and Proportions of T3 +, T4 + and T8 + Cells and T4/T8 Ratio in Study Groups
REFERENCES
1. Hadziyannis S, Maussonoous A, Viscoulis C, Afrondakis A. Cytoplasmic localization of Australia antigen in the liver. Lancet i:976. 1972.

2. Thomas HC. Cellular immunity to the hepatitis B virus. In: Bianchi L, Gerok W, Sickinger K, Stalder GA, eds. Virus and Liver. 161. Lancaster: MTP Press limited, 1980.
3. Thomas HC. The immunopathology of autoimmune and hepatitis B virusinduced chronic hepatitis. Semin Liver Dis 4:36. 1984.


4. Pignata G, Vajro P, Troncone R, Monaco G, Ciriaco M. Immunoregulatory T cell subsets in chronic active viral hepatitis: Characterization by monoclonal antibodies. J Pediatr Gastroenterol Nutr 2:229. 1983.


5. Burrai I, Carbone A. Subpopulation of T lymphocytes in patients with chronic active liver disease. Ric Clin Lab 12:501. 1982.

6. Thomas HC, Brown D, Routhier G, Janossy G, Kung PC, Goldstein G, Sherlock S. Inducer and suppressor T cells in hepatitis B virus-induced liver disease. Hepatol 2:202. 1982.

7. Hodgson HJF, Wands JR, Isselbach KJ. Alteration in suppressor cell activity in chronic active hepatitis. Proc Natl Acad Sci 75:1549. 1978.



8. Eddleston AL, Mondelli M. Immunopathological mechanisms of liver cell injury in chronic hepatitis B virus infection. J Hepatol 3:517. 1986.

9. Frazer IH, Mackay IR. T-lymphocyte subpopulations defined by two sets of monoclonal antibodies in chronic active hepatits and systemic lupus erythematosus. Clin Exp Immunol 50:107. 1982.


10. Hattum JV, Oudenaren AV, Schalm SW. T-lymphocyte subpopulations in patients with various courses after hepatitis B virus infection. Scand J Gastroenterol 19:497. 1934.

11. Park B, Koo JY, DeGroote J. Immunological aspects in patients with chronic active hepatitis. J Korean Medical Science 1:15. 1986.



12. Alexander GJM, Nouri-Aria KT, Eddleston ALWF, Williams R. Contrasting relations between suppressor-cell function and suppressor-cell number in chronic liver disease. Lancet i:1291. 1983.

13. Leevy CM, Popper H, Sherlock S. Diseases of the liver and biliary tract: Standardizatin of nomenclature, diagnostic criteria, and diagnostic methodology. Sponsored by the John E Fogarty International Centre for Advanced Study in Health Sciences and International Association for the Study of the Liver. Proceeding No. 22. 79ŌĆō7269ŌĆō11Washington DC: US Government Printing Office, DHEW Publication No (NIH). 1976.
14. Boyum O. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Invest Suppl 97:77. 1968.
15. Reinherz EL, Kung PC, Goldstein G, Schlossman SF. A monoclonal antibody with selective reactivity with functionally mature human thymocytes and all peripheral human T-lymphoctyes. J Immunol 123:1312. 1979a.

16. Reinherz EL, Kung PC, Goldstein G, Schlossman SF. Further characterization of the human inducer T-cell subset defined by monoclonal antibody. J Immunol 123:2894. 1979b.



17. Sakane T, Green I. Human suppressor T cells induced by concanavalin A: Suppressor T cells belong to distinctive T cell subclasses. J Immunol 119:1169. 1977.



18. Margolick JB, Fauci AS. Assays for suppressor cells. In: Rose NR, Friedman H, Fahey JL, eds. Manual of Clinical Laboratory Immunology. 3rd ed.. 315. Washington, DC: American Society for Microbiology, 1986.
19. DE Groote J, Desmet VJ, Gedigk P, Korb G, Popper H, Poulsen H, Scheuer PJ, Schmid M, Thaler H, Uehlinger E, Wepler W. A classification of chronic hepaitis. Lancet ii:626. 1968.


20. Vergani D, Portmann B, Eddleston AL, Williams R. Specificity of T lymphocyte cytotoxicity to autologous hepatocytes in chronic hepatitis B infection; evidence that T cells are directed against HBV core antigen expressed on hepatocytes. J Immnol 6:2773. 1982.
21. Van den Oord JJ, Desmet VJ. Verteilungsmuster der histokompatibilitatshauptantigene im normalen und pathologischen levergewebe. Leber Magen Darm 14:244. 1984.

22. Van den Oord JJ, De Vos R, Desmet VJ. In situ distribution of major histocompatibility complex products and viral antigens in chronic hepatitis B virus infection; Evidence that HBc-containing hepatocytes may express HLA-DR antigens. Hepatology 6:981. 1986.


23. Chisari FV, Castle KL, Xavier C, Anderson DS. Functional properties of lymphocyte subpopulations in hepatitis B virus infection. 1. Suppressor cell control of T lymphocyte responsiveness. J Immunol 126:38. 1981.

24. Brown WR, Stober W. Immunological diseases of the gastrointestinal tract. In: Samter M, Talmage DW, Frank MM, Austin KF, Claman HN, eds. Immunological Diseases. 4th ed. 1995. Boston/Toronto: Little, Brown and Company, 1988.
25. Thomas HC, Brown D, Labrooy J, Epstein O. T cell subsets in autoimmune and HBV-induced chronic liver disease, HBs antigen carriers with normal histology and primary biliary cirrhosis; a review of the abnormalities and the effect of treatment. J Clin Immunol 2:57s. 1982.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



