INTRODUCTION
Rickettsial diseases of humans consist of a variety of clinical entities caused by microorganisms of the family Rickettsiaceae. The rickettsias are obligate intracellular parasites about the size of bacteria and are usually seen microscopically as pleomorphic coccobacilli. Murine typhus fever is an acute febrile disease caused by Rikettsia typhi (mooseri) and transmitted to humans by fleas. The clinical illness is characterized by fever, skin rash, headache, and myalgia1).
Murine typhus has a world wide distribution. In Korea, there were not a few reports till 1960ŌĆÖs2). However, there have been no reports for two decades3ŌĆō5). Lee et al. reported cases of murine typhus with the help of immunoflorescence test after two decades of blanks6). We present this case to stress again the potential of laboratory-acquired infections and to alert the physician that murine typhus could be one of them.
CASE REPORT
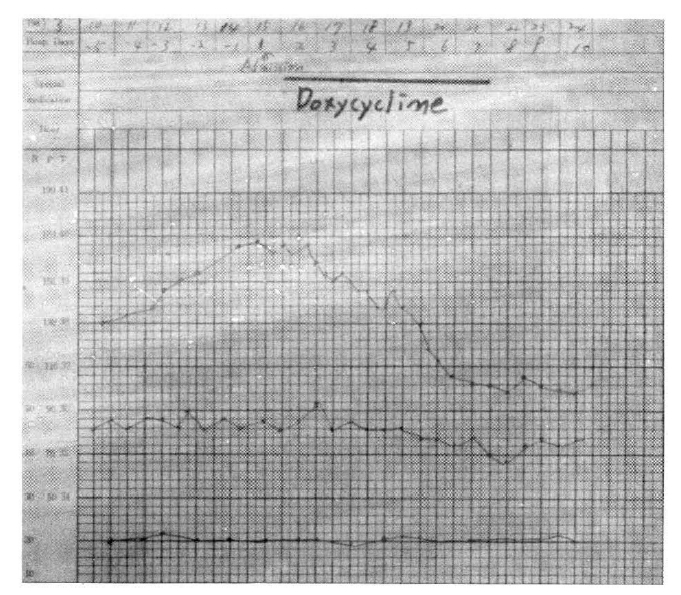
A 32-year-old Korean woman was admitted to the hospital for evaluation of fever, rash, and generalized myalgia. She worked as a technician in a laboratory for rickettsial disease (mouse inoculation and cell culture). The present illness began five days before admission, when she awoke with fever, chills, and generalized weakness. She visited a private clinic and was treated under the impression of upper respiratory infection, but her symptoms did not improve. For the next two days, she developed skin rashes on the thorax and abdomen. Her familial medical history was not contributory and past health had been excellent, with no previous hospitalizations, operations, or injuries. On physical examination, she was consciousnessly alert but in severe distress. Temperature was 39.8┬░C, blood pressure 130/80 mmHg, heart rate 90/min, and respiration 20/min (Fig. 1). Ill-defined maculopapular rashes had developed and spread on the thorax and back. Individual lesions consisted of pin-head sized erythematous macules with blanching margins (Fig. 2). There was no nuchal rigidity, no pale conjunctiva, and no icteric sclera. On auscultation, breathnig sounds were clear, and the heart sound was regular without murmur or friction rubs. Examination of the abdomen was unremarkable except for mild tenderness on both lateral aspects. The neurologic examinations were physiologic. On laboratory finding, hemoglobin was 10.0 gm/dl, hematocrit was 30.3%, white blood cell count was 1,700/mm3 with 23% polymorphonuclear neutrophils, 22% juveniles, and 48% lymphocytes. The platelet count was 95, 000/mm3 and ESR was 43 mm/hr. Urinalysis was negative for protein and revealed only 6ŌĆō8/HpF of WBC. On blood chemistry, BUN and creatinine concentrations were normal, serum bilirubin was 0.5 mg%, SGOT was 105 unit/L, SGPT was 80.2 unit/L, albumin was 3.2 gm%, globulin was 2.3 gm%. The electrocardiogram was normal. Radiologic examinations of the chest and abdomen were unremarkable (Fig. 3). The Widal test was negative, ASO test, the RA factor and ANA (antinuclear antibody) were negative. All cultures of blood, urine, and stool remained negative. Proteus agglutination titer (OX-2) was negative at 1 : 40 on day of admission. Immunofluorescence test with rickettsial antigen (R. typhi) was positive at 1 : 320 on admission and 1 : 1280 after 4 weeks, 1 : 40 after 16 weeks, and negative for R. tsutsugamushi. The patient was believed to have murine typhus on the basis of her clinical presentation, and treatment with 200 mg of doxycycline, administered orally at 24-hour intervals, was begun immediately. She complained of high fever, generalized aching, and skin rashes until the third hospital day. On the sixth hospital day, she became afebrile and the skin rashes began to fade. When antibiotics were discontinued 7 days later, there was no recrudescence of the disease. Arousal improved gradually and the patient appeared healthy.
DISCUSSION
In 1926, Maxcy published reports of epidemiological studies which indicated the rat to be a reservoir of infections. It remained for Dyer and co-workers, however, in 1931, to separate the louse-borne typhus of Europe from the sporadic typhus of Southeastern United States by demonstrating the rat flea to be the vector and the rat to be the reservoir host of these endemic cases of typhus7). Murine typhus, caused by Rickettsia typhi, is a small, gram negative, obligate, intracellular pathogen that shares common soluble antigens with R. prowazekii and R. canada8).
Murine typhus is found in all parts of the world. The disease occurs in those people whose occupation or living conditions bring them into close contact with rats and, therefore, the ectoparasites of these rodents9). In the rat, the disease is nonfatal. It is transmitted rat to rat by the rat flea (Zenopsylla cheopis) and possibly by the rat louse (Polypax spinnulosis) or other insects10). In the flea, the organism multiples in the cells of the digestive tract without harm to the insect. When the flea takes a blood meal, it defecates. The feces is heavily contaminated with organisms and produces infections in humans by soiling the bite wound. Scratching associated with the flea bite facilitates the inoculation of the infected feces into the bite site or skin abrasion11). Infection may also occur by mucous membrane (conjunctiva or nasal mucosa) contamination with flea feces or by aerosols inhaled by laboratory personnel9). Prevalence usually peaks in the late summer and fall7).
The clinical features of murine typhus are characterized by high fever, headache, myalgia after being biten by the rat flea or rat louse, and an incubation period lasting 8 to 16 days. Rash occurs in 60 percent of the patients, and it first becomes evident on the third to fifth day of illness12). The rash is initially macular occurring on the upper thorax and abdomen, and it remains central in distribution. This distribution is quite distinct from the primarily peripheral (ankles, wrists, and face) distribution of spotted fever. Later, the rashes of murine typhus become maculopapular and remain for 4 to 8 days9). In our case, the rashes were smaller in size than the typical ones. In most patients, a fever between 38.9┬░C and 40.0┬░C usually lasts 12 to 16 days. Other manifestations are cough, hemoptysis, hypotension, pulmonary congestion; also, acute febrile meningitis13) and neurologic sequelae develop in murine typhus14).
The serologic tests for murine typhus are the Weil-Felix reaction test, which is non-specific; the complement fixation (CF) test, indirect immunofluorescent antibody (IFA) test, ELISA, and the indirect immunoperoxidase test15), all of which are more specific. Among these tests, the indirect immunoflourescent test is the most specific and sensitive, but the indirect immunoperoxidase test is the most recommended, because it is inexpensive and can be read easily by light microscopy.
Tetracycline and chloramphenicol are both effective clinically in the treatment of all rickettsial diseases16). Tetracycline may be more effective than chloramphenicol in rapidly ameliorating the symptoms. Doxycycline is also effective11). Erythromycin is effective in vitro but has been disappointing in clinical practice. Rifampin is the most effective antibiotic in vitro17), but there are no clinical data.
Although the reported mortality with murine typhus is low (1 % to 2%), most of these deaths are said to occur in the aged. The morbidity can be substantial with prolonged disability and fever lasting an average of 15 days (range: 8 to 25 days) in untreated patients7,18).
To prevent murine typhus, control of the rat population is essential. This is best accomplished by poisoning and trapping, rat-proofing buildings, and locating garbage sites inaccessible to rats17). Chemoprophylaxis is not recommended for routine use in any situation. However, it may be useful in special situations, where adherence to a critical time-dose relationship unique for each rickiettsiosis can be assured. No murine typhus vaccine is currently available; murine typhus vaccines have been prepared in the past, but their efficacy has not been established19).
In summary, we experienced a case of murine typhus which occurred in a laboratory. The main causes of laboratory-acquired infections are faulty procedures in microbe handling or inadequate safeguards20). Therefore, all microbiology laboratory workers should be protected against infectious agents by use of protective clothing and rubber gloves and by working under a safety hood if possible.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





