 |
 |
| Korean J Intern Med > Volume 20(2); 2005 > Article |
|
Abstract
Inflammatory pseudotumor (plasma cell granuloma) of the lung is an uncommon nonneoplastic tumor of unknown origin. This tumor typically manifests as a solitary, peripheral, and sharply circumscribed mass. Multiple lesions are seen in about 5% of cases. Resection is recommended for both diagnosis and treatment, and this tumor does not generally recur after complete resection. Here, we report a case of recurrent inflammatory pseudotumor after complete resection; the recurrence was detected as a series of bilateral consolidated lesions with an internal air bronchogram. This is an unusual finding with regard to inflammatory pseudotumors.
Inflammatory pseudotumor (plasma cell granuloma) of the lung is an uncommon, nonneoplastic tumor of unknown origin. An aberrant or exaggerated response to tissue injury with no established cause has generally been thought to be the pathogenesis of this tumor1). Suspected lesions can be diagnosed as inflammatory pseudotumors on the basis of a combination of histologic features, including fibroblasts arranged in a storiform pattern and intermixed with variable numbers of plasma cells; small lymphocytes and histiocytes; central loose to dense collagen, infiltrated by plasma cells and histiocytes; or rarely, large lymphoid aggregates, some with germinal centers, separated by loose fibrous connective tissue. This tumor typically manifests as a solitary, peripheral, sharply circumscribed mass2). Multiple lesions are seen in 5% of cases3). Resection is recommended for both diagnosis and treatment, and recurrence is rare when these tumors are completely resected2,4-7).
In this study, we review the case of an inflammatory pseudotumor of the lung, which recurred after complete resection. Initially, the tumor manifested as a typical solitary, peripheral, and sharply circumscribed mass, but then recurred as a series of bilateral consolidated lesions, also including an internal air bronchogram. This represents a very rare finding with regard to inflammatory pseudotumors in general.
A 56-year-old man with no significant medical history was admitted to our hospital for a lung mass on August 3, 1998. He was a 30 pack-year smoker, and worked as a scavenger. He complained of chronic cough with purulent sputum, and weight loss of about 5 kg in the past year. Upon physical examination, no abnormalities were found. The plain chest radiograph and CT scan revealed a relatively well-defined soft mass in the left lower lobe of the lung (Figure 1). Transbronchial lung biopsy and percutaneous fine needle aspiration proved not to be diagnostic.
A left lower lobectomy was performed on August 14, 1998. Upon gross examination, a well-demarcated lobulated, solid mass, measuring 4.3×3.4 cm, was detected in the lateral basal segment of the left lower lobe. The tumor exhibited a grayish-yellow, focally fibrotic cut surface, with anthracitic pigmentation. Probing of the lateral basal segmental bronchus revealed an obstruction located 3.0 cm from the resection margin. Microscopic examination revealed this to be a circumscribed lesion without a capsule. The alveolar architecture was obliterated, with a small number of residual airways appearing at the periphery of the lesion. The lesion itself consisted of a mixture of fibroblasts, myofibroblasts, and inflammatory cells, especially plasma cells, lymphocytes, occasional eosinophils, and histiocytes. The spindle cells were arranged in a storiform pattern, and clusters of plasma cells were interspersed with the lymphocytes. Lymphoid follicles were also occasionally seen (Figure 2). The interlobar and inferior pulmonary ligament lymph nodes exhibited reactive hyperplasia. This lesion was diagnosed as an inflammatory pseudotumor, of the lymphoplasmacytic variety.
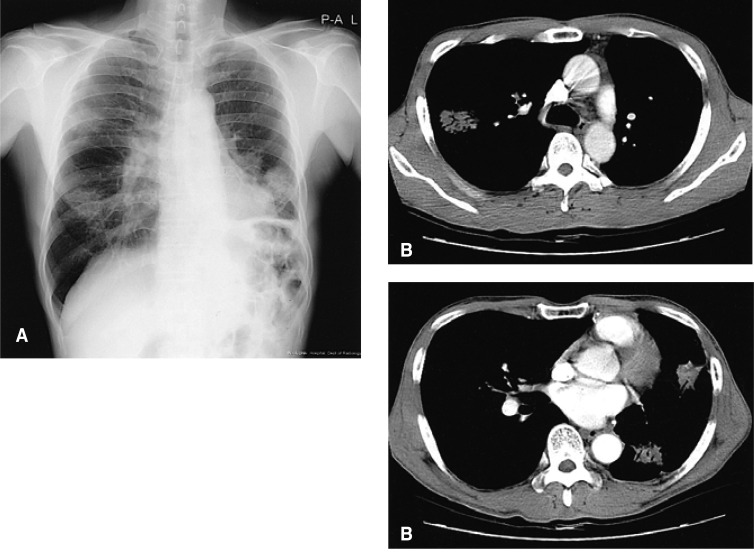
This patient returned to our hospital on December 26, 2002, complaining of aggravated cough and excessive sputum, which had persisted for 4 months. He also complained of intermittent hemoptysis. The plain chest radiograph and chest CT scan revealed three ill-defined consolidative lesions. One of these lesions was about 3.6 cm in size, with an internal air bronchogram, in the right upper lobe. The other lesions were about 3.5 cm in size, and they were located in the lingular division of the left upper lobe; these exhibited an appearance similar to that of the lesion in the right upper lobe (Figure 3). Ill-defined small nodules were also noted around these lesions. Transbronchial lung biopsy was again performed. The biopsy specimens through the posterior segment of the right upper lobe revealed marked interstitial lymphoplasmocytic infiltrations with fibrosis. Biopsies through the superior segment of the lingular division in the left upper lobe also revealed marked interstitial lymphoplasmocytic inflammation with fibroblast proliferation. Microscopically, the specimens also exhibited massive infiltrations of inflammatory cells, including plasma cells, lymphocytes, and neutrophils, admixed with fibroblasts and myofibroblasts. These findings were similar to those from the previous mass (Figure 4). We recommended a thoracoscopic biopsy, but the patient refused. We began oral prednisolone at 40 mg/day, and after gradually tapering the dosage, maintained it at 10 mg daily. We used this regimen for a total of 14 months, discontinuing it in March, 2004. On serial chest radiographs, all lesions appeared to have completely disappeared. On the patient's last visit, on August 31, 2004, the patient still complained of cough and sputum. There was, however, no evidence of a third recurrence on the follow-up chest radiograph.
Inflammatory pseudotumor, plasma cell granuloma, and inflammatory myofibroblastic tumor are all synonyms for a rare benign neoplasm which consists of histiocytes, myofibroblasts, plasma cells, and spindle-shaped mesenchymal cells7). The incidence of this tumor in the lung is between 0.04%4) and 0.7%8). This tumor is a nonneoplastic process, which is characterized by the unregulated growth of inflammatory cells. The cause of this particular condition remains unknown, although it has been suggested to be an immunologic response to a viral or foreign antigen-antibody reaction1,9,10). About 60% of these patients are symptomatic at diagnosis, and some have experienced a prior lower respiratory tract infection2). In our case, the patient complained of cough and sputum at the initial presentation, and then again when the tumor recurred. Inflammatory pseudotumors are subclassified into three histologic patterns: organizing pneumonia, fibrohistiocytic, and lymphoplasmacytic11). Our case was clearly of the lymphoplasmacytic variety, and we were able to diagnose it as a recurrence by comparing the transbronchial lung biopsy specimen to the lobectomy specimen.
In general, inflammatory pseudotumors do not recur after complete resection7). Forty-seven years of experience at Mayo clinic in the treatment of this tumor has revealed only 3 recurrences in a total of 23 cases, and all of these were due to incomplete resection4). In 67 children with inflammatory pseudotumors confined to the lung, the lesions recurred in only one case, and in that case, the tumor was enucleated instead of resected, whereas 8% of the extrapulmonary pseudotumors which were confined to a single organ recurred. Pseudotumors that were not confined to a single organ have a greater chance of recurrence--46% of pulmonary tumors, and 30% of extrapulmonary tumors. Therefore, inflammatory pseudotumors which, on initial presentation, are confined to a single organ, especially to the lung, recur only infrequently. Although tumor containment within a single organ is of great prognostic significance, complete removal with adequate margins is of equal importance7). A few reports in English medical literature, however, have chronicled recurrence after complete resection12,13). In our case, the tumor recurred after complete resection, even with sufficient resection margins.
According to a study by Agrons and colleagues2), inflammatory pseudotumors typically present as solitary, peripheral, sharply circumscribed masses, having an anatomic preference for the lower lobes. Forty-one patients (79%) with solitary parenchymal lesions exhibited sharply circumscribed nodules or masses. Twelve (20%) patients exhibited parenchymal lesions with ill-defined borders, and of these, four manifested as parenchymal consolidations. Three patients (5%) exhibited multiple parenchymal nodules, with no lobar predilection. Our case also initially involved a typical solitary, peripheral, sharply circumscribed mass. When the tumor recurred, it manifested as a set of bilateral, ill-defined consolidated lesions with internal air bronchogram, with ill-defined small nodules surrounding these main lesions. This is a very unusual finding with regard to this tumor. Only two reports in the English literature besides the study of Agrons and collegues2) described this tumor as an infiltrative lesion14) or a hazy shadow15).
Complete resection remains the key to prevention of disease recurrence. Patients with recurrent disease who are suitable for surgery should undergo reresections4). When the lesions prove impossible to remove surgically, or if they are not removed completely for any reason, corticosteroid therapy or radiation therapy was also performed14,16,17). In our case, reresection was ruled out as the lesions were bilateral, and corticosteroid treatment yielded good results.
Here, we have reported a case of inflammatory pseudotumor, which recurred after complete resection as a set of multiple, bilateral consolidated lesions with internal air bronchogram, and ill-defined small nodules surrounding the consolidations. Therefore, when encountering such an uncommon finding after the complete resection of pulmonary inflammatory pseudotumors, physicians should consider the possibility of pseudotumor recurrence.
References
1. Coffin CM, Dehner LP, Meis-Kindblom JM. Inflammatory myofibroblastic tumor, inflammatory fibrosarcoma, and related lesions: an historical review with differential diagnostic considerations. Semin Diagn Pathol 1998;15:102–110PMID : 9606802.

2. Agrons GA, Rosado-de-Christenson ML, Kirejczyk WM, Conran RM, Stocker JT. Pulmonary inflammatory pseudotumor: radiologic features. Radiology 1998;206:511–518PMID : 9457206.


3. Patankar T, Prasad S, Shenoy A, Rathod K. Pulmonary inflammatory pseudotumor in children. Australas Radiol 2000;44:318–320PMID : 10974727.


4. Cerfolio RJ, Allen MS, Nascimento AG, Deschamps C, Trastek VF, Miller DL, Pairolero PC. Inflammatory pseudotumors of the lung. Ann Thorac Surg 1999;67:933–936PMID : 10320231.


5. Altman H, Pietra GG, LiVolsi VA. Tracheobronchial inflammatory myofibroblastoma: a locally invasive, potentially recurrent neoplasm. Int J Surg Pathol 1994;2:93–98.

6. Dahabreh J, Zisis C, Arnogiannaki N, Katis K, Jubrail D, Charalamobos Z, Niki A, Konstantinos K. Inflammatory pseudotumor: a controversial entity. Eur J Cardiothorac Surg 1999;16:670–673PMID : 10647841.


7. Janik JS, Janik JP, Lovell MA, Hendrickson RJ, Bensard DD, Greffe BS. Recurrent inflammatory pseudotumors in children. J Pediatr Surg 2003;38:1491–1495PMID : 14577073.


9. Herman PG, Hillman B, Pinkus G, Harris GC. Unusual noninfectious granulomas of the lung. Radiology 1976;121:287–292PMID : 981599.


10. Shirakusa T, Miyanzaki N, Kitagawa T, Sugiyama K. Ultrastructural study of pulmonary plasma cell granuloma: report of a case. Br J Dis Chest 1979;73:289–296PMID : 553662.


11. Matsubara O, Tan-Liu NS, Kenney RM, Nark EJ. Inflammatory pseudotumor of the lung: progression from organizing pneumonia to fibrous histiocytoma or plasma cell granuloma in 32 cases. Hum Pathol 1988;19:807–814PMID : 2841219.


12. Yokomura K, Chida K, Suda T, Kuwata H, Suzuki K, Matsuda H, Asada K, Nakamura Y, Inui N, Tsuchiya T, Suzuki K, Nakamura H. Recurrence of inflammatory pseudotumor of the lung with endobronchial lesion after resection. Nihon Kokyuki Gakkai Zasshi 2002;40:129–134PMID : 11974867.

13. Kim JH, Cho JH, Park MS, Chung JH, Lee JG, Kim YS, Kim SK, Kim SK, Shin DH, Choi BW, Choe KO, Chang J. Pulmonary inflammatory pseudotumor: a report of 28 cases. Korean J Intern Med 2002;17:252–258PMID : 12647641.



14. Diez Pina JM, Fernandez Vazquez E, Saez Roca G, Canizares Sevilla F, Marin Aznar JL, Lopez la Osa A. Multifocal inflammatory pseudotumor of the lung with good response to corticoids. Arch Bronconeumol 1998;34:102–104PMID : 9580519.


15. Bandoh S, Ueda Y, Namihira H, Dohmoto K, Fujita J, Takahara J, Okada H, Maeda M, Yamadori I. Inflammatory pseudotumor of the lung. Nihon Kokyuki Gakkai Zasshi 1999;37:504–508PMID : 10434553.

Figure 1
The plain chest radiograph displays a relatively well-defined solitary mass in the left lower lobe.
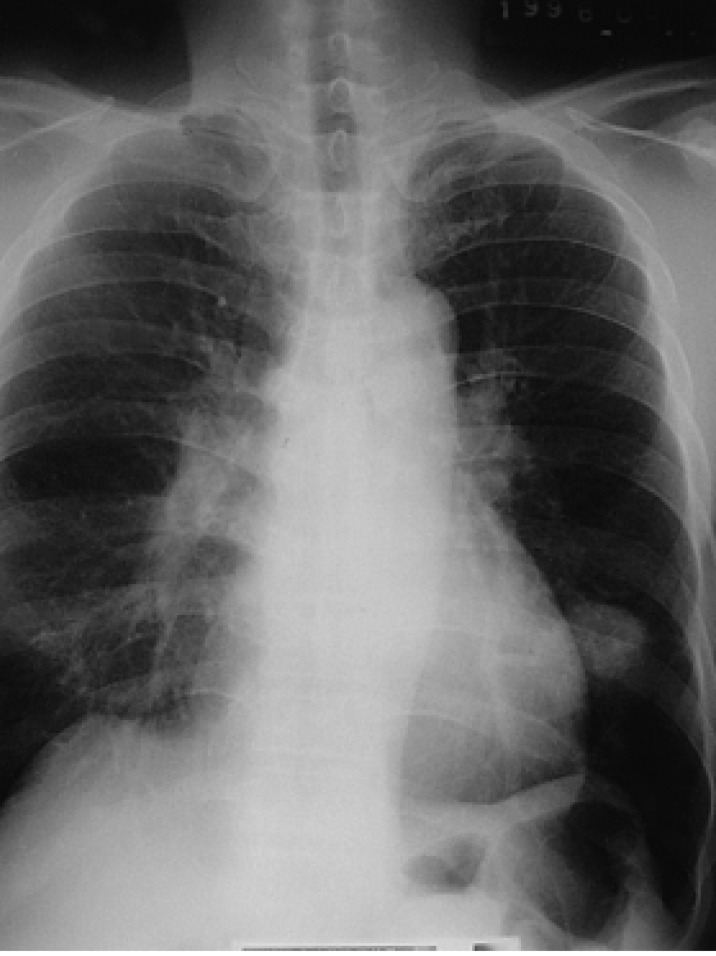
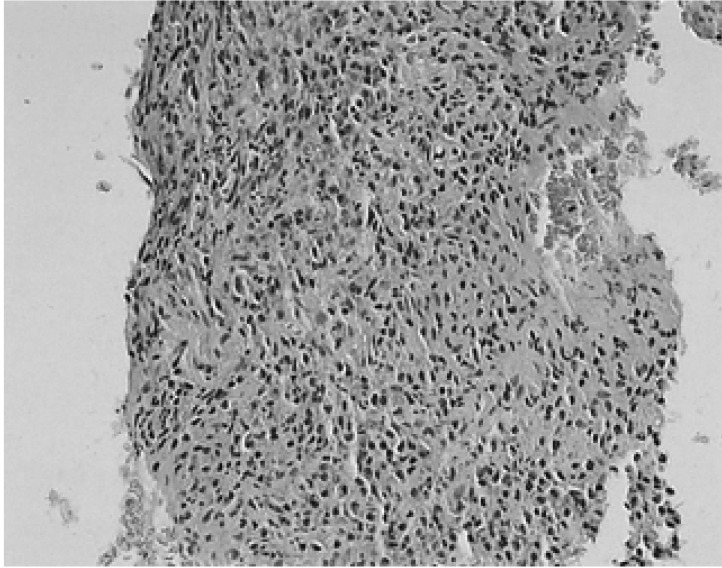
Figure 2
Microscopic findings of inflammatory pseudotumor. The alveolar architecture is obliterated. The spindle cells are arrayed in a storiform pattern, and interspersed with many inflammatory cells with small lymphoid follicles (H & E, ×40) (A). The picture shows a mixture of spindled fibroblasts, myofibroblasts, and inflammatory cells, primarily plasma cells, lymphocytes, and occasional eosinophils. No nuclear atypia or mitotic figures are evident (H & E, ×400) (B).




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



