 |
 |
| Korean J Intern Med > Volume 27(3); 2012 > Article |
|
Abstract
Background/Aims
The recently published Oxford classification of IgA nephropathy (IgAN) proposed a split system for histological grading, based on prognostic pathological features. This new classification system must be validated in a variety of cohorts. We investigated whether these pathological features were applicable to an adult Korean population.
Methods
In total, 69 adult Korean patients with IgAN were analyzed using the Oxford classification system at Soonchunhyang University Hospital, Seoul, Korea. All cases were categorized according to Lee's classification. Renal biopsies from all patients were scored by a pathologist who was blinded to the clinical data for pathological variables. Inclusion criteria were age greater than 18 years and at least 36 months of follow-up. We excluded cases with secondary IgAN, diabetic nephropathy combined other glomerulopathies, less than 36 months of follow-up, and those that progressed rapidly.
Results
The median age of the patients was 34 years (range, 27 to 45). Mean arterial blood pressure was 97 ± 10 mmHg at the time of biopsy. The median follow-up period was 85 months (range, 60 to 114). Kaplan-Meier analysis showed significant prognostic predictions for M, E, and T lesions. A Cox proportional hazard regression analysis also revealed prognostic predictions for E and T lesions.
IgA nephropathy (IgAN) is the most common form of primary glomerular disease worldwide [1-3]. IgAN is defined by the presence of mesangial IgA immune deposits in the absence of any systemic disorder [4,5]. The clinical and histological features of IgAN are highly variable. IgAN may present with a variety of histological patterns, ranging from minimal lesions to diffuse proliferative and crescentic glomerulonephritis [6-8]. Numerous clinicopathological studies of IgAN have correlated histological changes in diagnostic biopsies with clinical outcomes [6,9,10]. Each of these classifications, developed according to expert opinions, has strengths and limitations in terms of their prognostic predictions, but none is considered preeminent [11]. A working group of the International IgA Nephropathy Network and Renal Pathology Society proposed the new "Oxford classification" of IgAN, which includes four pathological variables: mesangial hypercellularity (M0/M1 lesion), segmental glomerulosclerosis (S0/S1 lesion), endocapillary hypercellularity (E0/E1 lesion), and tubular atrophy/interstitial fibrosis (T0/T1/T2 lesion) [8,11]. This new classification needs to be validated in different cohorts. We conducted this study to assess the pathological variables of the new Oxford classification in an adult Korean population.
Patients who were followed for more than 3 years (before December, 2010) at the Soonchunhyang University Hospital, Seoul, Korea, were included in the study sample. From January 1994 to December 2007, data were collected from renal biopsies of native kidneys performed and/or processed in our unit. The diagnosis of IgAN was defined by the predominant mesangial deposition of IgA. All patients were older than 18 years at the time of biopsy. All cases had been reported according to Lee's classification [12].
Cases with secondary causes of mesangial IgA deposits, such as Henoch-Schonlein purpura, and those with comorbid conditions, such as diabetes mellitus, were excluded. Patients with less than 36 months of follow-up were also excluded to maximize the reliability of the estimation of renal function decline. Rapidly progressive cases were also excluded. The definition of "rapidly progressive cases" included patients with a decreasing estimated glomerular filtration rate (eGFR) > 50% within 6 months. Two cases were excluded because their conditions were complicated by systemic diseases; one patient had pneumonia, and the other presented with an acute kidney injury after an operation.
A eGFR was determined using the four-variable modification of diet in renal disease formula for adults [13,14]. Mean arterial blood pressure (MAP) was defined as diastolic pressure plus one-third of the pulse pressure. Immunosuppressive treatment was reported as intent to treat, regardless of the type or duration of therapy. Renin-angiotensin (RAS) blockade indicated any exposure to an angiotensin-converting enzyme inhibitor and/or angiotensin receptor blocker. Two different clinical outcomes were studied to address the predictive values of pathological variables: 1) the rate of renal function decline (slope of eGFR), and 2) survival with a 50% reduction in renal function, or end-stage renal disease (ESRD). The slope of eGFR was calculated as follows: difference between initial eGFR and last eGFR divided by the number of years, in the same way as the working group (unit, mL/min/1.73 m2/yr) [11].
Continuous variables are expressed as means ± standard deviations or medians and ranges. Nominal data are presented as percentages. Comparisons among multiple groups were performed using the Mann-Whitney, Kruskal-Wallis, or Spearman tests. Categorical variables were compared using the Pearson χ2 test. Univariate analysis followed by multiple linear regression was used to determine independent predictors of eGFR slope. Cumulative renal survival from the time of renal biopsy was analyzed using Kaplan-Meier curves for censored data with each histological variable. Univariate Cox regression was performed to test the association between each pathological variable and renal events on the influence of baseline clinical prognostic factors (protein excretion, hypertension, eGFR). The same models were studied through multivariate Cox regression analysis with univariate significant pathological variables. All statistical analyses were performed using the SPSS version 14.0 (SPSS Inc., Chicago, IL, USA). On two-sided tests, p values < 0.05 were considered to indicate statistical significance.
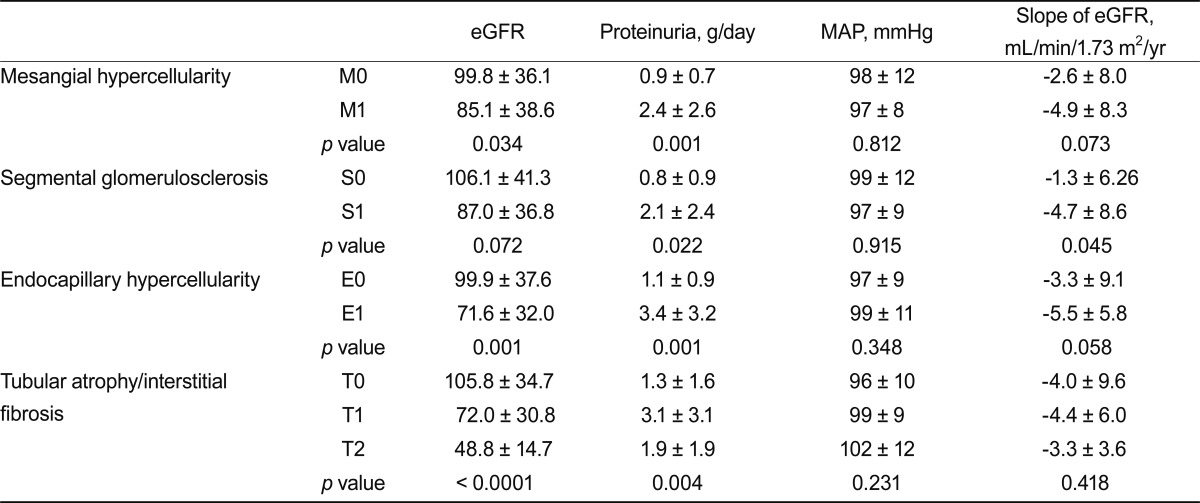
Clinical features at the time of biopsy are shown in Table 1. At the time of renal biopsy, the median age was 34 years (range, 27 to 45), with a male predominance. Mean MAP was 97 ± 10 mmHg and mean eGFR was 90.8 ± 38.0 mL/min/1.73 m2. Four subjects took a hypertensive medication (one took diuretics, three took calcium channel blockers). Median follow-up duration was 85 months (range, 60 to 114). The distribution of histological lesions according to the Oxford classification was as follows: M1, 42 (60%); S1, 55 (80%); E1, 22 (32%); and T1/T2, 17/8 (25/12%). According to Lee's classification, the distribution of grades was as follows: grade 1, 9 (13%); grade 2, 9 (13%); grade 3, 19 (27%); grade 4, 26 (37%); and grade 5, 6 (10%). Endocapillary hypercellularity and tubular atrophy/interstitial fibrosis were correlated with proteinuria and decreased eGFR at the time of biopsy (Table 2).
Patients with E1 or T1/T2 lesions were more likely to have been treated with immunosuppressants. RAS blockade was prescribed for 92% of patients, and 17% of the cohort was treated with fish oil (Table 3).
Among our cohort, 6 (9%) patients reached a 50% reduction in eGFR and 10 (14%) patients progressed to ESRD. The cumulative probabilities of ESRD or a 50% reduction in initial eGFR in patients with grade IV and V lesions versus those with grade III (p = 0.014, 0.001) according to Lee's classification are shown in Fig. 1. When grade I/II/III and grade IV/V were categorized, grade IV/V was associated with a significantly increased relative risk of ESRD or a 50% reduction in eGFR by Cox regression (hazard ratio, 2.27; confidence interval, 1.381 to 4.422; p = 0.037).
By the univariate pathological variables of the Oxford classification, linear regression of rate of renal function decline correlated only with S lesions (p = 0.045). M, E, and T lesions showed no significant correlation with the rate of renal function decline (p = 0.073, 0.058, and 0.418, respectively) (Table 2). In multiple linear regression analyses, S and E lesions also failed to show any correlation with the rate of renal function decline.
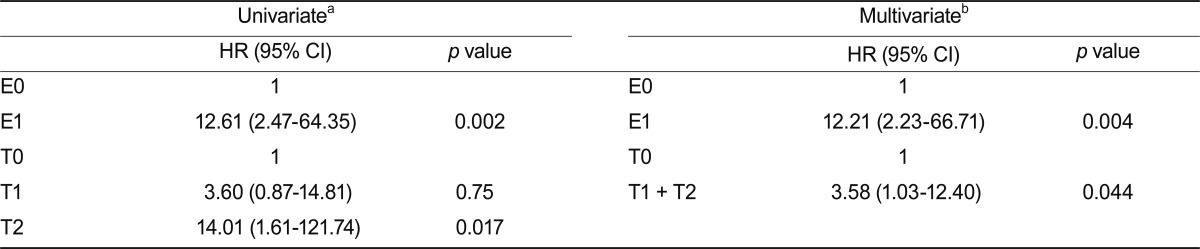
Kaplan-Meier analyses showed that M1, E1, and T1 lesions were associated with either ESRD or a 50% reduction in initial eGFR. No significant difference was found between T2 and T1 lesions (Fig. 2). Univariate Cox regression analysis using pathological variables and a clinical data set (eGFR, proteinuria, and MAP) showed that E and T lesions were predictive of renal outcome. Multivariate analysis with two pathological features, which were validated in the univariate analysis, were calculated to address whether biopsy findings predicted long-term outcome independently of the initial assessment (clinical data set: eGFR, MAP, and proteinuria). This analysis revealed that only E lesions had prognostic significance (Table 4). When we merged the T1 and T2 lesion data to maximize statistical power, T lesions (tubular atrophy/interstitial fibrosis, > 25%) were also predictive of renal outcome (Table 4).
A recent international effort identified a set of distinct pathological variables with prognostic value [11]. This classification has since been validated in a few independent cohorts [4,15]. In this study, we assessed the value of each pathological variable of the new Oxford classification for IgAN for prediction of renal outcome in Korean adults. Many studies have attempted to predict renal outcomes using various histological grading systems [9,10,16,17].
Previously used pathological systems [6] for the classification of renal lesions in IgAN can be subdivided into two types: lumped and split. Lumped systems assess the overall severity of histological lesions found in the glomerular, tubular, interstitial, and arteriolar compartments, as in the widely used classification of Lee and Haas [6,10]. This is simple and readily applicable to multicenter studies, but the weakness of a lumped system is the lack of flexible interpretation. Split systems, such as the Oxford classification, use semi-quantitative grading of lesion severity in each of the four compartments of the kidney and permit the elaboration of a global or aggregate score for each compartment [18]. No strategy for prognostic prediction from biopsy-based pathological evaluations has yet been established due to the lack of reproducibility and inappropriate categorization [8,11,19].
The working group of the Oxford classification may offer important advances by providing evidence that not only chronic fibrotic change, but also mesangial and endocapillary hypercellularity, predict prognosis [11]. The working group reported that when the rate of eGFR decline was included as a dependent variable in multivariate linear regression models, S and T lesions, but not M lesions, were associated with renal outcomes. However, when a 50% decrease in eGFR or ESRD was included as a dependent variable in the Cox proportional hazards model, M and T lesions, but not S lesions, were found to be significant predictors of renal outcome [11].
In our cohort, interactions between each pathological lesion and treatment modalities, such as RAS blockers or immunosuppressive agents, were assessed only in univariate models. The working group of the Oxford classification showed that patients with E lesions were exposed to significantly more immunosuppressants than patients without such findings [11]. Thus, they hypothesized that E lesions might respond to immunosuppressive treatment and so offered indirectly the possibility of prognostic value [11]. However, we found that E and T lesions had significant value in predicting the reduction of eGFR or ESRD, independent of clinical parameters and other pathological variables, even though patients with E lesions received more immunosuppressive agents (Tables 3 and 4). Additionally, M lesions in the Kaplan-Meier analysis, and S lesions had significant predictive value in predicting renal function decline. Mesangial hypercellularity and segmental glomerulosclerosis were associated with initial proteinuria. However we did not consider the treatment response during follow-up, which may explain the inconsistency.
We enrolled cases with more than 36 months of follow-up to maximize statistical power. In our cohort, Lee's classification provided information for predicting renal outcome in univariate analyses. However, with respect to histological lesions on a continuous scale of risk assessment, not every stage increased the risk of adverse renal outcome in the Cox regression analysis. Regarding clinical decisions with this lumped system, there is a lack of relevance to pathological variables. This new classification system, specifically E and T lesions, predicted renal outcomes in our population independent of clinical data in our study population (Table 4). Histopathological classifications should be developed to predict not only renal outcomes, but also responsiveness to treatment modalities. Whether it is beneficial to base clinical decisions on this classification system, in particular, the novel parameters of mesangial and endocapillary hypercellularity, should be verified in a prospective study with a large sample size.
The Oxford classification was studied as a prognosis predictor in another university hospital in Korea. The findings were slightly different from those of our study. Kang et al. [20] reported that M lesions were associated with proteinuria and the number of antihypertensive drugs. Their results indicated that T, but not M, lesions were associated with survival from ESRD or of having a 50% reduction in eGFR. The inconsistent results of multivariate models for predicting renal outcome might be partly due to the retrospective study design, and assessment of pathological variables could be subjective, due to the single pathologist, in both studies.
The value of this study is that we demonstrated that these pathological features have prognostic and/or predictive value in Korean adults. A previous report indicated that endocapillary proliferation had a lesser impact on renal outcomes in East Asians than in patients of European descent [11]. This observation was not consistent with our findings. However, the sample population and patient age differed between the two studies. The international consensus working group for the IgAN study included Chinese and Japanese subjects, 48 adults and 14 children; in our study, all subjects were Korean adults.
A study that compared the lesions in renal biopsies of children and adults using the Oxford classification found greater mesangial hypercellularity and endocapillary proliferation in children and greater glomerular sclerosis, chronic interstitial damage, and arteriolar change in adults [21]. Another study revealed that proliferative lesions were more likely to respond to treatment in children than in adults [19]. These observational studies suggested that patient age could affect pathological features in predicting renal outcome and responsiveness to treatment.
Many of our findings were unexpected; in particular, no significant difference between T1 and T2 lesions in terms of prediction of renal outcomes was found. However, the Kaplan-Meier curve showed a tendency towards differences in survival between patients with T1 and T2 lesions (Fig. 2). These findings could be related to the low statistical power; only eight patients in our sample had T2 lesions.
The major limitation of this study is its retrospective observational nature; retrospective studies are susceptible to selection bias. Thus, clinical and pathological data for our cohort were from the time of biopsy; we could not consider the treatment response factor during follow-up. We included a limited number of cases, and all data were collected from a single center. Additionally, a single pathologist analyzed all of the material according to the Oxford classification; thus, the reproducibility of the system was not validated.
In conclusion, this study indicated that the pathological variables of the Oxford classification system were useful in Korean adults with IgAN. Such information could be used to provide individualized treatment decisions.
References
1. Neelakantappa K, Gallo GR, Baldwin DS. Proteinuria in IgA nephropathy. Kidney Int 1988;33:716–721PMID : 3367561.


2. Alamartine E, Sabatier JC, Guerin C, Berliet JM, Berthoux F. Prognostic factors in mesangial IgA glomerulonephritis: an extensive study with univariate and multivariate analyses. Am J Kidney Dis 1991;18:12–19PMID : 2063844.


3. Walsh M, Sar A, Lee D, et al. Histopathologic features aid in predicting risk for progression of IgA nephropathy. Clin J Am Soc Nephrol 2010;5:425–430PMID : 20089495.



4. Coppo R. Pediatric IgA nephropathy: clinical and therapeutic perspectives. Semin Nephrol 2008;28:18–26PMID : 18222343.


5. Coppo R, Gianoglio B, Porcellini MG, Maringhini S. Frequency of renal diseases and clinical indications for renal biopsy in children (report of the Italian National Registry of Renal Biopsies in Children): group of renal immunopathology of the Italian Society of Pediatric Nephrology and group of renal immunopathology of the Italian Society of Nephrology. Nephrol Dial Transplant 1998;13:293–297PMID : 9509437.


6. Haas M. Histologic subclassification of IgA nephropathy: a clinicopathologic study of 244 cases. Am J Kidney Dis 1997;29:829–842PMID : 9186068.


7. Ibels LS, Gyory AZ. IgA nephropathy: analysis of the natural history, important factors in the progression of renal disease, and a review of the literature. Medicine (Baltimore) 1994;73:79–102PMID : 8152367.


8. Working Group of the International IgA Nephropathy Network and the Renal Pathology Society. Roberts IS, Cook HT, et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int 2009;76:546–556PMID : 19571790.


9. Wakai K, Kawamura T, Endoh M, et al. A scoring system to predict renal outcome in IgA nephropathy: from a nationwide prospective study. Nephrol Dial Transplant 2006;21:2800–2808PMID : 16822793.


10. Lee HS, Lee MS, Lee SM, et al. Histological grading of IgA nephropathy predicting renal outcome: revisiting H. S. Lee's glomerular grading system. Nephrol Dial Transplant 2005;20:342–348PMID : 15618239.


11. Working Group of the International IgA Nephropathy Network and the Renal Pathology Society. Cattran DC, Coppo R, et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int 2009;76:534–545PMID : 19571791.


12. Lee SM, Rao VM, Franklin WA, et al. IgA nephropathy: morphologic predictors of progressive renal disease. Hum Pathol 1982;13:314–322PMID : 7076216.


13. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999;130:461–470PMID : 10075613.


14. Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145:247–254PMID : 16908915.


15. Herzenberg AM, Fogo AB, Reich HN, et al. Validation of the Oxford classification of IgA nephropathy. Kidney Int 2011;80:310–317PMID : 21544062.


16. Manno C, Strippoli GF, D'Altri C, Torres D, Rossini M, Schena FP. A novel simpler histological classification for renal survival in IgA nephropathy: a retrospective study. Am J Kidney Dis 2007;49:763–775PMID : 17533019.


17. Kobayashi Y, Tateno S, Hiki Y, Shigematsu H. IgA nephropathy: prognostic significance of proteinuria and histological alterations. Nephron 1983;34:146–153PMID : 6348567.


18. Wyatt RJ, Emancipator SN, Kon V, et al. IgA nephropathy databank: development of a system for management of renal biopsy acquired data. Am J Kidney Dis 1997;29:817–828PMID : 9186067.


19. Haas M, Rahman MH, Cohn RA, Fathallah-Shaykh S, Ansari A, Bartosh SM. IgA nephropathy in children and adults: comparison of histologic features and clinical outcomes. Nephrol Dial Transplant 2008;23:2537–2545PMID : 18263928.


Figure 1
Kaplan-Meier plot of renal survival according to Lee's classification. ap = 0.014, bp = 0.001.
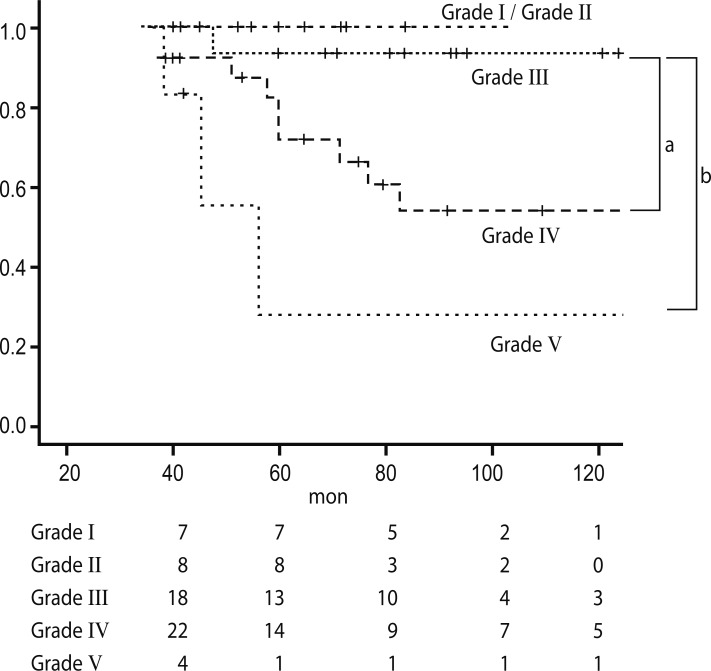
Figure 2
Kaplan Meier plot of renal survival according to pathological features of the Oxford classification.
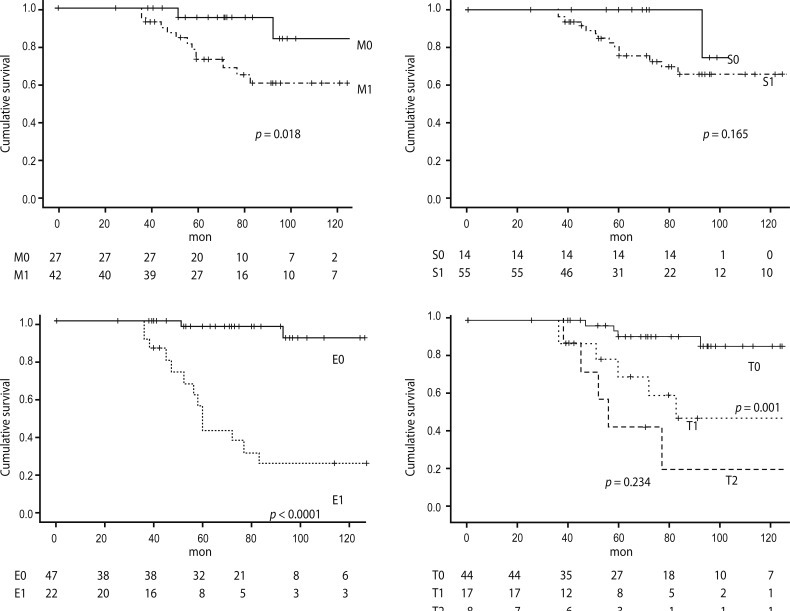
Table 1
Clinical characteristics and pathological features at the time of biopsy in 69 patients with IgA nephropathy and clinical data of follow-up
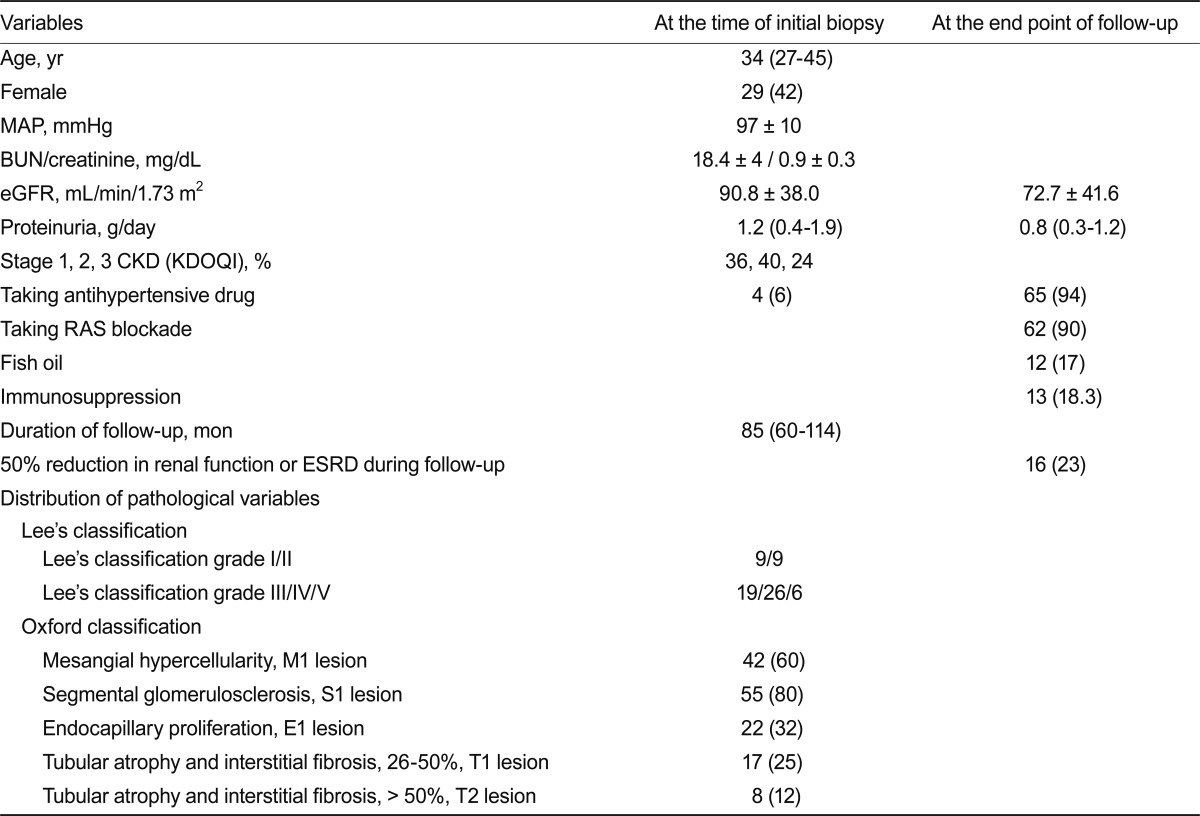
Table 2
Correlations between pathological and clinical features at the time of renal biopsy and the rate of renal function decline during follow-up




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



