 |
 |
| Korean J Intern Med > Volume 27(4); 2012 > Article |
|
To the Editor,
The Epstein-Barr virus (EBV) is an ubiquitous human herpesvirus that mainly infects B-cells. After primary infection, EBV can induce both replicative (productive or lytic) and latent (persistent) infections in lymphocytes. Latent EBV infection is linked to the development of severe chronic active EBV (SCAEBV) infection with T-cell/natural killer cell (T/NK-cell) lymphoproliferative disorder (LPD) [1]. SCAEBV infection with T-cell LPD is characterized by persistent or recurrent infectious mononucleosis (IM)-like symptoms: high titers of anti-EBV antibodies, the presence of EBV genomes in affected tissues, including peripheral blood, chronic illness that cannot be explained by other known diseases, and monoclonal proliferation of EBV-infected T-cells [2]. In here, we report a case of SCAEBV infection in which T-cell LPD was confirmed by lung biopsy with video-assisted thoracic surgery (VATS) in an adult Korean patient with a history of follicular lymphoma (FL).
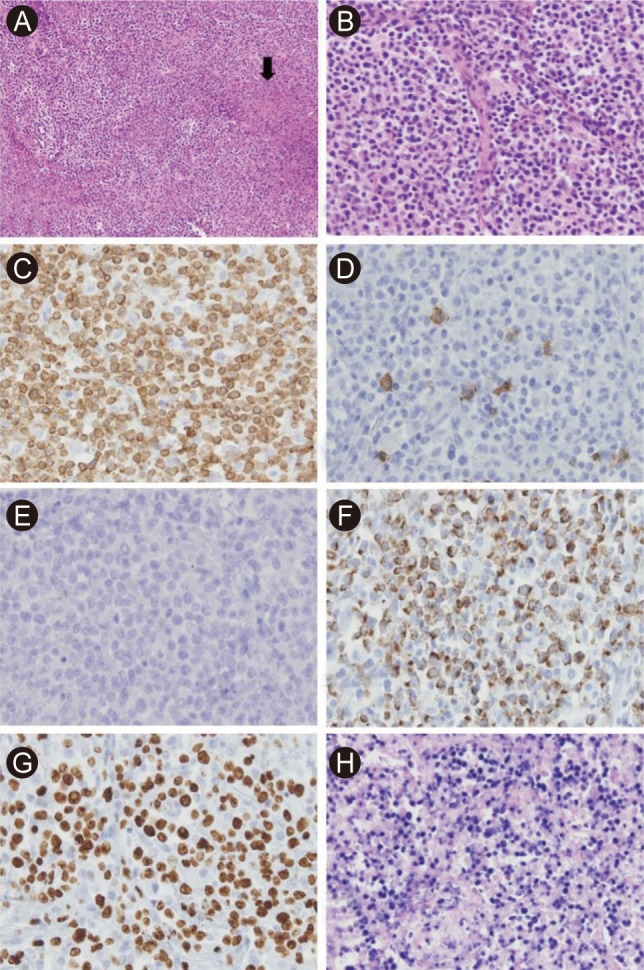
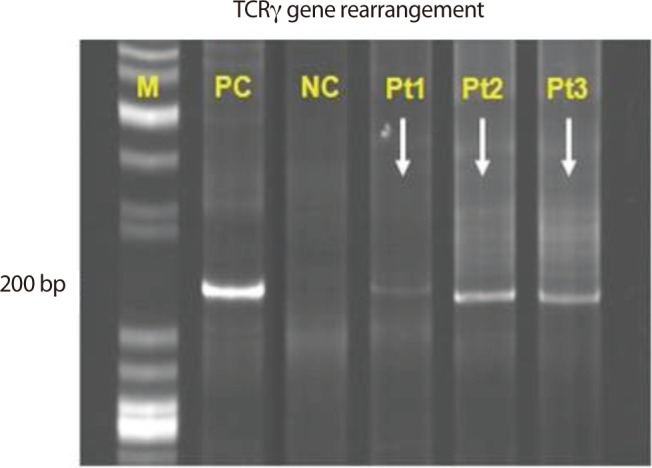
A 57-year-old man was admitted to Korea Cancer Center Hospital with the chief complaint of intermittent fever for four months. He had lost 5 kg weight over a period of 2 months and had a sore throat, night sweating, and intermittent uveitis. Approximately 4 years prior to his admission to our hospital, he was diagnosed with FL (histologic grade 3, Ann Arbor stage IIA) involving both tonsils and both internal jugular chain lymph nodes. A complete response (CR) was achieved after six cycles of therapy with rituximab, cyclophosphamide, vincristine, and prednisolone followed by radiation therapy, and the CR status was maintained by the patient for 3 years. Therefore, we initially suspected FL recurrence or FL transformation to diffuse large B-cell lymphoma (DLBL). Imaging studies revealed the presence of multiple small, nodular ground-glass opacities (GGOs) in the upper lobes of both lungs and hepatosplenomegaly (Fig. 1A). A blind liver biopsy was performed using a biopsy gun to confirm FL recurrence; however, the pathological test results revealed chronic hepatitis without infiltration of FL cells. Therefore, lung biopsy with VATS was performed for a lesion in the right upper lobe of the lung. The lung biopsy revealed multifocal patchy lymphohistiocytic infiltrations in the tissue; the majority of the infiltrating cells were CD3-positive T-cells. There was no evidence of FL recurrence. The patient was administered steroids under the impression of interstitial pneumonitis and was discharged when the symptoms improved. However, the symptoms recurred after temporary improvement, and the patient was readmitted 2 months after the first hospitalization. On admission, his vital signs were normal. There was no evidence of cervical lymphadenopathy or abnormal findings in the chest and abdomen. The peripheral leukocyte count was 6,420/µL, and the hemoglobin level was 11.8 g/dL. The results of the serologic tests were as follows: anti-hepatitis B surface antibody (+), anti-hepatitis C virus antibody (-), EBV viral capsid antigen (VCA)-immunoglobulin G (IgG) (+), EBV VCA-IgM (-), EBV nuclear antigen IgG (+), EBV early antigen (EA) IgG (+). Real-time polymerase chain reaction (RT-PCR) analysis for EBV-DNA quantification from peripheral blood monocyte cells revealed an EBV-DNA level of 15.5 copies/µL (normal range, < 3.8). A peripheral blood smear revealed microcytic normochromic red blood cells with anisocytosis and poikilocytosis. Bone marrow (BM) sections displayed normocellular marrow with a normal distribution of nucleated cells and no evidence of BM involvement in FL. Chest computed tomography (CT) demonstrated that the multifocal nodular GGOs in both lungs had increased in size and number in comparison with those observed during the first hospitalization, and a new nodular mass with a size of 2 cm had developed in the right lower lobe (RLL) of the lung (Fig. 1B). A second lung biopsy with VATS was performed for a new mass in the RLL of the lung. Microscopically, the lung mass displayed numerous atypical lymphoid cell infiltrations in the alveolar spaces and interstitium of the tissue. The infiltrates were mainly composed of medium-sized T-cells. The tumor mass contained multifocal necrotic and apoptotic areas (Fig. 2A and 2B). Immunohistochemical staining demonstrated that the tumor cells were positive for CD3 and granzyme B, but negative for CD8, CD56, CD4, CD10, and CD20 (Fig. 2C-2F). Most cells were Ki-67-positive proliferating cells (Fig. 2G). In situ hybridization with EBV-encoded RNA confirmed the presence of EBV in a majority of tumor cells (Fig. 2H). T-cell receptor gamma (TCRγ) gene rearrangement showed a monoclonal pattern (Fig. 3). Therefore, the final pathologic diagnosis was EBV-positive T-cell LPD. Additional EBV in situ hybridization was performed on the previously obtained lung and liver biopsy tissues, and focally positive EBV expression was noted in the lymphocytic infiltrates of the lung tissue and in a few lymphocytes in the sinusoids of the liver biopsy tissue. However, the lung and liver biopsies did not reveal lymphocytic atypia or tumorus histology. The patient received chemotherapy with cytarabine, methylprednisolone, doxorubicin, and cisplatin (ASHAP). Chest CT scan after one cycle of ASHAP demonstrated that the multifocal nodular GGOs in both lungs had decreased in size and number. The patient then received a second cycle of ASHAP. However, he developed pneumonia and died because of progressive pneumonia and septic shock.
There are two different types of chronic active EBV (CAEBV) infections: chronic EBV infection, which displays persistent IM-like illness with a relatively good prognosis, and SCAEBV infection, which displays rather severe manifestations and generally poor prognosis with many life-threatening complications such as EBV-associated malignant lymphoma and hemophagocytic syndrome [3]. Recently, the following criteria for CAEBV infection diagnosis were proposed: 1) persistent or recurrent IM-like symptoms that persist for at least 6 months; 2) an unusual expression pattern of anti-EBV antibodies with elevated levels of anti-VCA and anti-EA and/or detection of increased EBV genomes in affected tissues, including the peripheral blood; and 3) chronic illness that cannot be explained by other known diseases at diagnosis [2]. Several clinical, hematologic, and pathological findings of our patient satisfied the CAEBV infection criteria for the following reasons: the patient experienced unexplained symptoms, including persistent fever, sore throat, malaise, and hepatosplenomegaly for at least 6 months, and displayed a high EBV antibody titer and elevated viral loads determined by RT-PCR. Pathological examination of the lung mass revealed many atypical lymphoid cell infiltrations and diffuse positivity for EBV in in situ hybridization experiments.
Ohshima et al. [1] suggested that EBV-infected T/NK-cells develop from polyclones or oligoclones and subsequently expand as monoclones to cause aggressive diseases such as lymphomas or hemophagocytic syndrome. In Korea, Joo et al. [4] recently reported a case of CAEBV infection with interstitial pneumonitis in a young woman. She recovered spontaneously and exhibited a good clinical course. In that case, TCRγ gene rearrangement exhibited a polyclonal pattern, unlike that observed in our case. That finding indicates that the case reported by Joo et al. [4] was a case of CAEBV infection that had not progressed to monoclonal LPD. Typically, EBV-associated LPDs are derived from B-cell diseases, such as Hodgkin's lymphoma or Burkitt's lymphoma, and are a well-established phenomenon in patients with primary or secondary immunodeficiencies [2]. However, SCAEBV infection with T/NK-cell LPD is usually observed in apparently immunocompetent individuals [2]. We believe our patient was relatively immunocompetent because he had a sufficiently long treatment-free interval in the CR state despite a history of FL. SCAEBV infection with T/NK-cell LPD is characterized by persistent EBV infection in T- or NK-cells and clonal proliferation of these EBV-positive populations for long periods. It is still not known why these EBV-infected cells proliferate in an uncontrolled manner in an apparently immunocompetent host. Kimura [5] hypothesized that EBV-infected T- or NK-cells can evade the cellular immune system of the host via limited expression of viral proteins and reduced antigenicities. The prognosis of SCAEBV infection remains very poor [1]. No effective chemotherapy has been reported for the treatment of adult-onset SCAEBV infection. Antiviral therapy and immunomodulatory agents are usually ineffective for treating SCAEBV infection. However, allogeneic hematopoietic stem cell transplantation (HSCT) appears to be a promising treatment option for SCAEBV infection [2].
We report a case of SCAEBV infection with T-cell LPD that was diagnosed by lung biopsy in a patient who had received chemotherapy and radiotherapy for FL and had maintained a CR state. Although there have been reports describing SCAEBV infection with T-cell LPD, this is the first case of SCAEBV infection with T-cell LPD in a Korean patient who had a history of B-cell lymphoma. Because adult-onset SCAEBV infection is more aggressive [1], the suspicion of SCAEBV infection is important if adult patients have chronic or recurrent IM-like symptoms such as intermittent low-grade fever, hepatosplenomegaly, and pancytopenia. Early recognition and diagnosis of SCAEBV infection, which is a borderline condition with a high risk of progression into aggressive NK- or T-cell lymphoma, may allow the implementation of beneficial treatment strategies. In cases of SCAEBV infection with LPD, allogeneic HSCT can be considered early in the disease course when the patient is better able to tolerate transplantation.
References
1. Ohshima K, Kimura H, Yoshino T, et al. Proposed categorization of pathological states of EBV-associated T/natural killer-cell lymphoproliferative disorder (LPD) in children and young adults: overlap with chronic active EBV infection and infantile fulminant EBV T-LPD. Pathol Int 2008;58:209–217PMID : 18324913.


2. Kawa K, Sawada A, Sato M, et al. Excellent outcome of allogeneic hematopoietic SCT with reduced-intensity conditioning for the treatment of chronic active EBV infection. Bone Marrow Transplant 2011;46:77–83PMID : 20498651.


3. Kimura H, Ito Y, Kawabe S, et al. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocompromised hosts: prospective analysis of 108 cases. Blood 2012;119:673–686PMID : 22096243.


Figure 1
(A) Chest computed tomography performed in September 2010 revealed multiple small nodular ground glass opacities in both upper lobes of the lung. (B) A new nodular mass with a size of 2 cm had developed in the right lower lobe of the lung in November 2010.
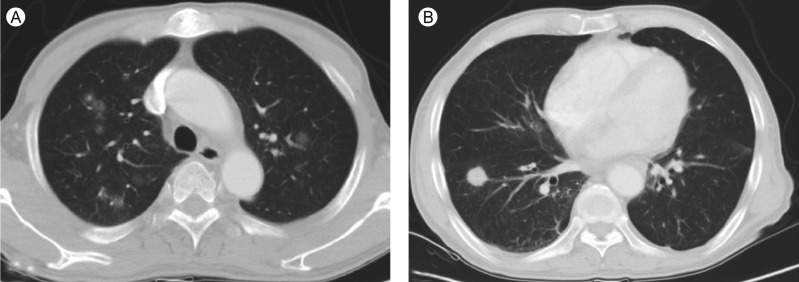
Figure 2
(A) Microscopically, the lung biopsy revealed heavy infiltration of lymphoid cells with a small patchy necrotic area inside the mass (arrow; H&E, × 100). (B) The infiltrates were mainly composed of medium-sized lymphoid cells in the alveolar space (H&E, × 250). Immunohistochemical analysis demonstrated that the infiltrating cells were CD3-positive (C), CD8-negative (D), CD56-negative (E), granzyme B-positive (F) T-cells. The proliferation marker Ki-67 (G) confirmed the high proliferation index (× 400). (H) Most tumor cells were Epstein-Barr virus-positive, as observed by EBV-encoded RNA (EBER) in situ hybridization (× 250).
-
METRICS

-
- 0 Crossref
- 1 Scopus
- 9,780 View
- 90 Download
- Related articles
-
A Case of Hemolytic Uremic Syndrome Associated with Epstein - Barr Virus Infection1998 July;13(2)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


