INTRODUCTION
Klotho is a single-pass transmembrane protein that was originally identified as an anti-aging protein in 1997 [
1], and was discovered to have various biological activities thereafter. The Klotho protein is not only present on the cell membrane, but is also secreted. The membrane-bound Klotho protein functions as an obligate co-receptor for fibroblast growth factor 23, and acts on the kidney to regulate negative phosphate balance [
23]. The secreted Klotho protein functions as a humoral factor that regulates the activity of multiple ion channels and growth factor receptors [
456].
Although Klotho is expressed in multiple organs, it is highly expressed in the kidney, especially in the distal convoluted tubules [
1], and plays various physiological roles in the development of acute kidney injury (AKI) and in the progression to chronic kidney disease (CKD) [
56789]. Sugiura et al. [
10] demonstrated in rats that renal Klotho mRNA and protein levels were significantly reduced in ischemia-reperfusion injury (IRI) on the first day after ischemia, but gradually recovered over the next 10 days. Hu et al. [
11] showed that AKI is a state of acute and transient Klotho deficiency in the blood, urine, and kidneys in the IRI animal model, and that urinary Klotho levels in patients with AKI were lower than those of healthy humans, suggesting that Klotho may have diagnostic and therapeutic potential in human AKI. However, there is still little evidence on the biological role of Klotho in human AKI, because most studies have used the animal IRI model. In human AKI studies, although urinary Klotho may be representative of renal Klotho, its expression has only been evaluated in urine, and not in injured tissues.
Considering the multiple causes of AKI, the role of renal Klotho needs to be investigated in patients with non-ischemic as well as ischemic AKI. In the present study, we used immunohistochemical staining to examine Klotho expression in the kidneys of patients with AKI, and evaluated its correlation with AKI severity and clinical outcome.
METHODS
Patients and data collection
The present study was a retrospective analysis of patients with AKI who were diagnosed with acute tubular necrosis (ATN) or acute tubulointerstitial nephritis (ATIN) by histological examination between January 2001 and December 2012 at the Korea University Hospital. The study was approved by the Institutional Review Board of the Korea University Anam Hospital (IRB no., ED12232).
We reviewed patients' medical records and collected data regarding sex, age, the underlying disease, the cause of AKI, the pathology report of the renal biopsy, and changes in creatinine level (the initial, peak, lowest, 1 month and final creatinine levels) during the follow-up period. In addition, data regarding the requirement for renal replacement therapy (RRT), death, and functional recovery were collected. The recovery of renal function was defined as a serum creatinine level < 1.3 mg/dL or an estimated glomerular filtration rate (eGFR) within 10% of baseline during the follow-up period. The eGFR was assessed on the basis of creatinine clearance, which was calculated using the Modification of Diet in Renal Disease GFR equation.
Histological quantification of Klotho
Formalin-fixed and paraffin-embedded tissues of patients were evaluated. All of the kidney biopsies were examined by a nephropathologist. Patients with chronic lesions-such as tubular atrophy, interstitial fibrosis, and glomerulosclerosis-were excluded from the study.
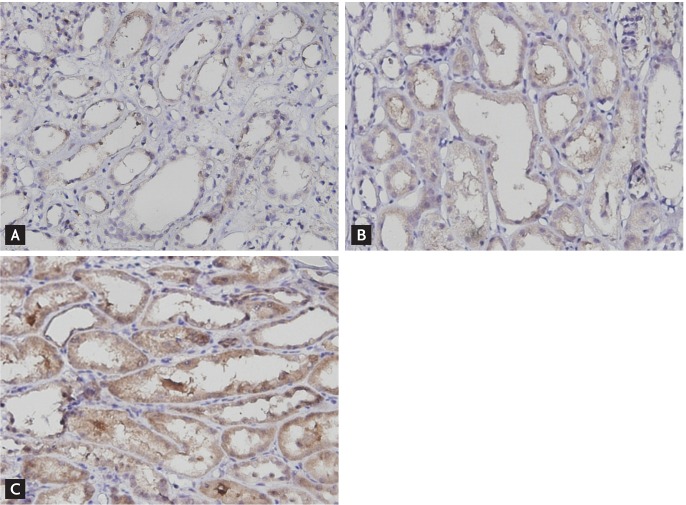
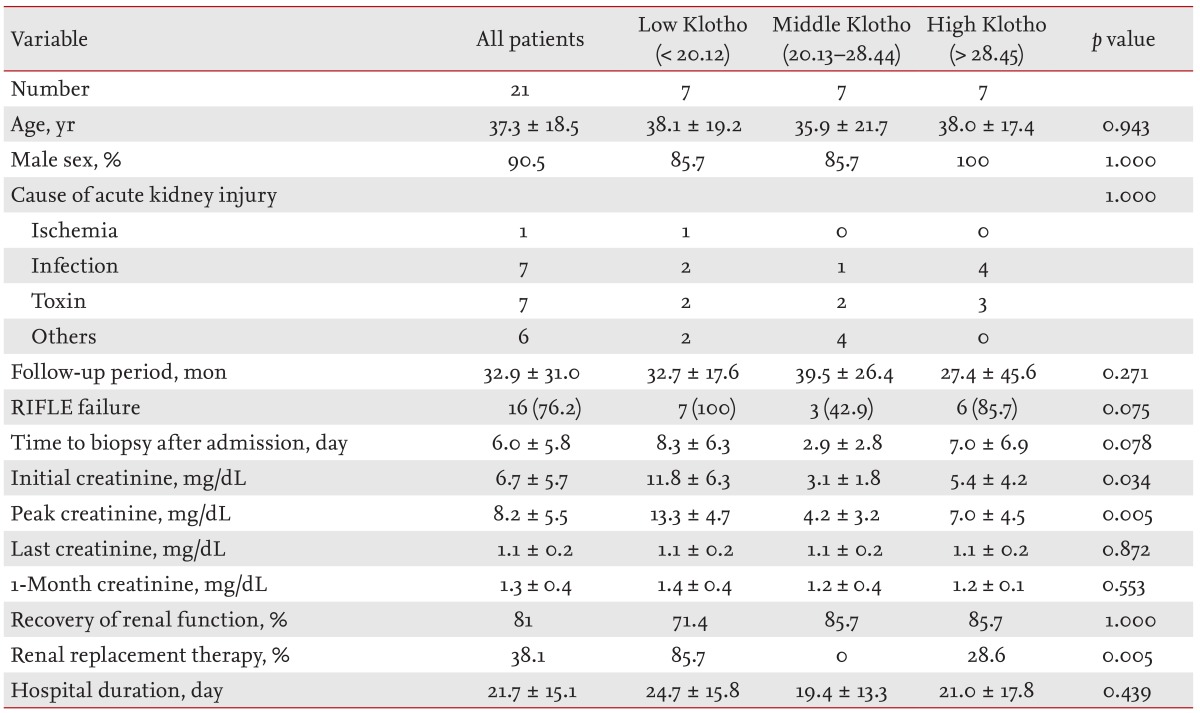
Immunohistochemical staining was performed according to the manufacturer's protocol. Briefly the sections were incubated with a rabbit antihuman Klotho polyclonal antibody (1:250 dilution, ab69208, Abcam, Hong Kong) for 45 minutes, and then incubated with an horseradish peroxidase-conjugated goat polyclonal antibody to rabbit immunoglobulin G as the secondary antibody. Klotho expression was quantified by measuring the intensity of immunohistochemical staining for Klotho using an image analyzer (Leica Application Suite, Leica Microsystems, Wetzlar, Germany). Klotho intensity was scored as a percentage of Klotho-positive pixels, by assessing all fields of the tissue, with the exception of the medulla, under a 200× magnification. In addition, the patients were divided into three equal groups according to the Klotho score (low, middle, or high). A Klotho score < 20.12 was defined as low, > 28.45 was defined as high, and 20.13 to 28.44 was defined as medium (
Fig. 1).
Statistical analysis
All of the statistical analyses were performed using SPSS version 20 (IBM Co., Armonk, NY, USA). Data are presented as mean ± standard deviation. Comparisons between groups were performed using Student t test or the Mann-Whitney test to compare two groups, an analysis of variance or Kruskal-Wallis test to compare three groups in the case of numerical data, and a chi-square test or Fisher exact test in the case of categorical data. The Pearson correlation or Spearman rank correlation analysis were performed to assess correlations. Multivariate multiple linear or logistic regression analysis was performed. A p < 0.05 was considered statistically significant.
DISCUSSION
It has been suggested that AKI may reflect a transient deficiency of renal Klotho, and this deficiency might be an important pathological feature in AKI development and progression to CKD. Reduction of renal Klotho protein in a rodent IRI-AKI model precedes the increase in creatinine or neutrophil gelatinase-associated lipocalin, suggesting that Klotho is one of the earliest biomarkers of AKI [
11]. Previous study also suggested a possible role for Klotho as a predictor of the progression of AKI to CKD [
12]. Moreover, the use of recombinant Klotho for treatment in an IRI rat model attenuated renal failure [
11], and the administration of an adenovirus-mediated Klotho gene to mice with IRI retarded the increase in the serum creatinine level and prevented renal histological damage [
10], indicating the potential of Klotho as a therapeutic agent.
On the basis of these results of previous animal studies, renal Klotho is expected to play important diagnostic and therapeutic roles in AKI; however, there has been little research on Klotho in AKI patients. This may be due to the fact that patients with AKI are usually diagnosed on the basis of clinical data rather than the results of histological examination, and are usually managed with conservative treatment, regardless of the cause. In addition, AKI includes a broad disease spectrum with heterogeneous clinical features, which can lead to difficulties in understanding the precise pathophysiology and performing tissue-based clinical research. To date, only a single human study of Klotho expression in AKI has been reported. In that study, urinary Klotho levels in patients with AKI were lower than those of healthy human controls, suggesting an important role for Klotho in human AKI [
11]. Urinary Klotho levels might reflect renal Klotho expression level. Previous animal studies showed parallel reductions in renal and urinary Klotho in mice and rats with AKI [
11]. However, despite its abundant expression in the kidney, renal Klotho expression has not been assessed in human AKI. Although there are some limitations in terms of the precise quantification of Klotho levels while performing histological examination, our study demonstrated, for the first time, renal Klotho expression in AKI patients by means of immunohistochemical staining.
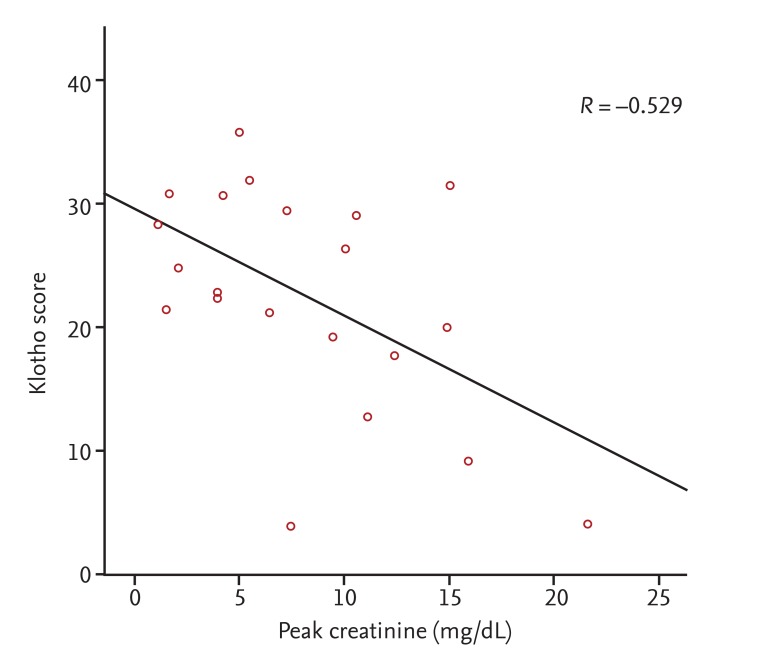
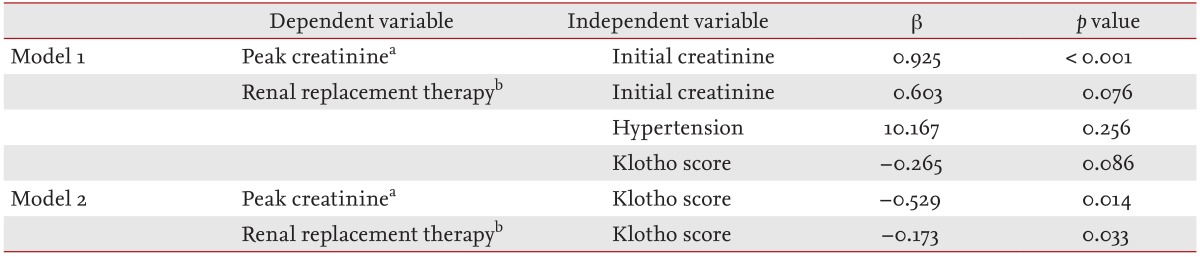
Interestingly, low renal Klotho expression was significantly correlated with AKI severity and poor short-term outcomes, such as the need for temporary RRT, although the initial creatinine level showed a stronger correlation. Almost all of the kidney biopsies in our patients were performed between 3 and 7 days after admission according to the clinical decision of whether the cause of AKI was unclear or if renal function was not improved despite treatment. In particular, the time to biopsy after admission in the middle Klotho group was shorter than that in the other Klotho groups (
Table 1), which could be associated with a relatively low expression of Klotho; this may have resulted in confusion in data interpretation. In fact, the high Klotho group tended to exhibit higher peak creatinine and a greater requirement for RRT than the middle Klotho group. Despite this limitation, the significant negative correlation of renal Klotho expression with the initial or peak creatinine level suggests that renal Klotho expression decreases significantly at some point after the AKI insult, regardless of the etiology and despite different sampling times. Therefore, the tissue level before 7 days reflects AKI severity early in the course of the disease. Although the present study cannot confirm the time course of changes in renal Klotho levels, previous animal studies have reported decreases within 3 hours after injury, with a return to baseline levels being dependent on the injury type and severity [
1011]. In a nephrotoxic animal model, renal Klotho mRNA levels markedly decreased up to 7 days after nephrotoxic insult, even when renal function had recovered [
12]. This delayed recovery of renal Klotho levels suggests that it may be a promising biomarker of progression to CKD as well as for the early detection of AKI.
In addition, the significant correlation between renal Klotho levels and renal function in our results suggests a possible renoprotective role for renal Klotho in AKI. In previous studies of the physiological roles of Klotho, its administration or gene delivery reduced kidney damage and decreased renal fibrosis through inhibition of apoptosis and transforming growth factor-β signaling [
7910]. In addition to the results of animal experiments, our findings in human AKI suggest a possible basis for further study regarding the clinical application of Klotho in AKI.
Klotho might also be considered a useful histological marker in AKI. In general, histological examination is performed for the differential diagnosis of AKI; however, it does not typically provide prognostic information about the clinical course because there is often a discrepancy between the pathologic data and clinical severity. Therefore, a tissue marker to quantify AKI severity and predict its outcome would be helpful in making treatment decisions. In the present study, reduced Klotho expression reflected severe functional deterioration. However, the renal Klotho level did not show a significant association with renal recovery. Future large-scale studies including patients who developed CKD after AKI are required to define the predictive role of Klotho. In addition, extended follow-up data on renal or urinary Klotho levels might be more helpful in predicting AKI outcomes, because previous reports have shown sustained Klotho deficiency during the recovery phase in animal models of AKI and a significant correlation between Klotho level and renal function in CKD [
121314].
This is the first human clinical study to identify the relationship between the renal Klotho level and AKI severity. However, this study had several limitations. First, the small scale and retrospective study design meant that the clinical value of renal Klotho in AKI could not be confirmed. Second, the timing of biopsy was heterogeneous, which could have resulted in considerable variation in the Klotho score. Nevertheless, the degree of reduction in the renal Klotho level showed a significant association with AKI severity. Third, immunohistochemical scoring is not a precise quantitative measurement. In the histological analysis, severe tubular damage and subsequently reduced tubular mass might be associated with low intensity of Klotho, although previous animal studies demonstrated that damaged tubules express a low level of Klotho. Therefore, to confirm that the low Klotho level reflects reduced tubular expression, quantification of the protein or gene expression levels in an adequate number of human specimens is necessary. Finally, most of the patients included in this study recovered from AKI and did not progress to CKD during the follow-up period. Therefore, we cannot definitively state the relevance of the Klotho level to progression to CKD.
In conclusion, human renal Klotho expression decreased significantly according to the severity of AKI, regardless of the etiology. Low Klotho expression was associated with a poor short-term outcome. These findings are consistent with previous studies in which Klotho deficiency accentuated AKI [
10], which suggests that Klotho deficiency may be a biomarker of renal injury. Future, large-scale prospective studies are necessary to define the clinical value of Klotho as a biomarker and to determine its potential as a therapeutic for AKI.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


