 |
 |
| Korean J Intern Med > Volume 30(5); 2015 > Article |
|
Abstract
Background/Aims:
Methods:
Results:
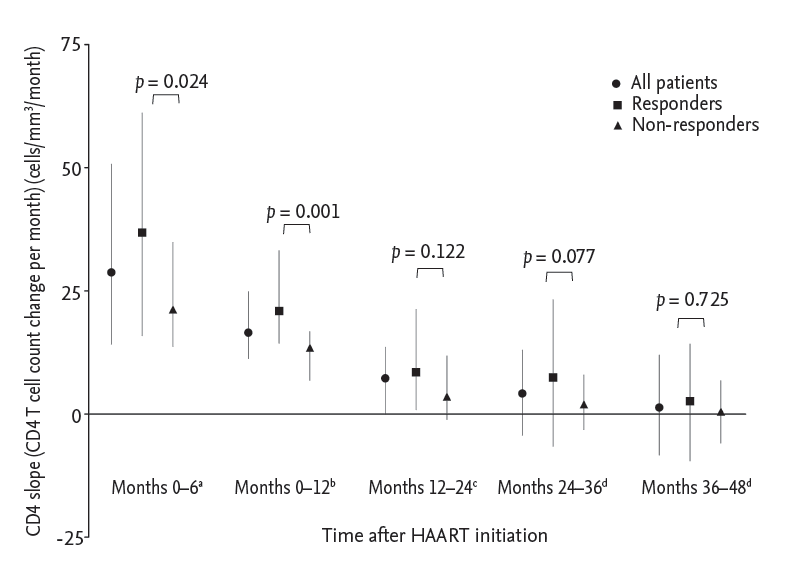
Figure┬Ā1.
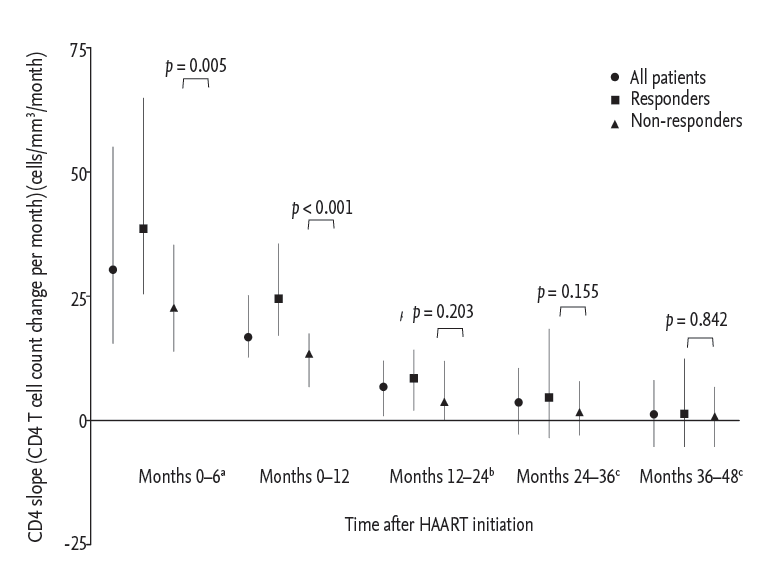
Figure┬Ā2.
Table┬Ā1.
| Characteristic | Responder (n = 59) | Non-responder (n = 43) | p value |
|---|---|---|---|
| Age, yr | 44.9 ┬▒ 9.3 | 44.8 ┬▒ 10.8 | 0.969 |
| Male sex | 49 (83.1) | 38 (88.4) | 0.454 |
| Route of HIV transmission | 0.328 | ||
| ŌĆāHeterosexual contact | 34 (57.6) | 28 (65.1) | |
| ŌĆāMale homosexual contact | 25 (42.4) | 14 (32.6) | |
| ŌĆāTransfusion | 0 | 1 (2.3) | |
| Time from diagnosis of HIV infection to initiation of HAART, day | 572.5 ┬▒ 895.2 | 746.6 ┬▒ 1,312.8 | 0.428 |
| Baseline CD4 T cell/mm3 | 173.4 ┬▒ 113.1 | 70.1 ┬▒ 69.6 | < 0.001 |
| Baseline CD4 T cell count < 200/mm3 | 33 (55.9) | 40 (93.0) | < 0.001 |
| Baseline CD4 T cell count < 50/mm3 | 10 (16.9) | 23 (53.5) | < 0.001 |
| Baseline viral load > 100,000 copies/mLa | 49 (83.1) | 35 (81.4) | 0.829 |
| CDC HIV-1 disease categoryb | 0.157 | ||
| ŌĆāA | 30 (50.8) | 15 (34.9) | |
| ŌĆāB | 11 (18.6) | 7 (16.3) | |
| ŌĆāC | 18 (30.5) | 21 (48.8) | |
| Hepatitis B or C coinfection | 6 (10.2) | 5 (11.6) | 0.815 |
| Syphilis | 30 (50.8) | 22 (51.2) | 0.975 |
| Opportunistic infections at the start of HAARTc | 20 (33.9) | 22 (51.2) | 0.080 |
| ŌĆāTuberculosis | 8 (13.6) | 9 (20.9) | 0.324 |
| ŌĆāPneumocystis pneumonia | 7 (11.9) | 8 (18.6) | 0.343 |
| ŌĆāCytomegalovirus disease | 2 (3.4) | 2 (4.7) | 0.746 |
| ŌĆāCryptococcal meningitis | 0 | 3 (7.0) | 0.039 |
| ŌĆāEsophageal candidiasis | 1 (1.7) | 2 (4.7) | 0.383 |
| Treatment-naïve when starting HAART | 57 (96.6) | 42 (97.7) | 0.753 |
| HAART regimend | 0.443 | ||
| ŌĆāUnboosted PI-based | 20 (33.9) | 13 (30.2) | |
| ŌĆāBoosted PI-based | 17 (28.8) | 18 (41.9) | |
| ŌĆāNNRTI-based | 16 (27.1) | 7 (16.3) | |
| ŌĆāMixed | 6 (10.2) | 5 (11.6) | |
| HAART regimen including ZDV | 51 (86.4) | 39 (90.7) | 0.510 |
| TMP/SMX use | 29 (49.2) | 32 (74.4) | 0.010 |
| Duration of TMP/SMX use, mon | 0.001 | ||
| ŌĆā< 1 | 31 (52.5) | 14 (32.6) | |
| ŌĆā1ŌĆō6 | 15 (25.4) | 6 (14.0) | |
| ŌĆā7ŌĆō12 | 10 (16.9) | 6 (14.0) | |
| ŌĆāŌēź 13 | 3 (5.1) | 17 (39.5) |
Values are presented as mean ┬▒ SD or number (%).
HIV, human immunodeficiency virus; HAART, highly active antiretroviral therapy; CDC, Centers for Disease Control and Prevention; PI, protease inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; ZDV, zidovudine; TMP/SMX, trimethoprim/sulfamethoxazole.
b According to the CDC classification [19].
c Defined as the surveillance case definitions for HIV infection and acquired immunodeficiency syndrome (AIDS) of CDC [19].
d According to the backbone antiretroviral drugs. The boosted PI-based regimen is defined as a ritonavir boosted PI-containing regimen. The mixed regimen is defined as switching from one class to another [19].
Table┬Ā2.
| Characteristic | Responder (n = 33) | Non-responder (n = 40) | p value |
|---|---|---|---|
| Age, yr | 44.0 ┬▒ 7.3 | 44.7 ┬▒ 11.1 | 0.754 |
| Male sex | 27 (81.8) | 35 (87.5) | 0.530 |
| Route of HIV transmission | 0.328 | ||
| ŌĆāHeterosexual contact | 17 (51.5) | 26 (65.0) | |
| ŌĆāMale homosexual contact | 16 (48.5) | 13 (32.5) | |
| ŌĆāTransfusion | 0 | 1 (2.5) | |
| Time from HIV diagnosis to HAART, day | 545.4 ┬▒ 1,017.6 | 737.8 ┬▒ 1,346.8 | 0.490 |
| Baseline CD4 T cell/mm3 | 89.7 ┬▒ 57.8 | 57.1 ┬▒ 51.3 | 0.013 |
| Baseline CD4 T cell count < 50/mm3 | 10 (30.3) | 23 (57.5) | 0.114 |
| Baseline viral load > 100,000 copies/mL | 31 (93.9) | 33 (82.5) | 0.171 |
| CDC HIV-1 disease categorya | 13 (39.4) | 12 (30.0) | 0.647 |
| ŌĆāA | 13 (39.4) | 12 (30.0) | |
| ŌĆāB | 4 (12.1) | 7 (17.5) | |
| ŌĆāC | 16 (48.5) | 21 (52.5) | |
| Hepatitis B or C coinfection | 2 (6.1) | 5 (12.5) | 0.446 |
| Syphilis | 16 (48.5) | 18 (45.0) | 0.766 |
| Opportunistic infections at the start of HAARTb | 9 (27.3) | 20 (50.0) | 0.048 |
| ŌĆāTuberculosis | 7 (21.2) | 9 (22.5) | 0.895 |
| ŌĆāPneumocystis pneumonia | 7 (21.2) | 8 (20.0) | 0.898 |
| ŌĆāCytomegalovirus disease | 2 (6.1) | 2 (5.0) | 1.000 |
| ŌĆāCryptococcal meningitis | 0 | 3 (7.5) | 0.247 |
| ŌĆāEsophageal candidiasis | 0 | 2 (5.1) | 0.498 |
| Treatment-naïve when starting HAART | 32 (97.0) | 39 (97.5) | 1.000 |
| HAART regimenc | 0.677 | ||
| ŌĆāUnboosted PI-based | 11 (33.3) | 12 (30.0) | |
| ŌĆāBoosted PI-based | 10 (30.3) | 16 (40.0) | |
| ŌĆāNNRTI-based | 9 (27.3) | 7 (17.5) | |
| ŌĆāMixed | 3 (9.1) | 5 (12.5) | |
| HAART regimen including ZDV | 28 (84.8) | 36 (90.0) | 0.723 |
| TMP/SMX use | 27 (81.8) | 31 (77.5) | 0.650 |
| Duration of TMP/SMX use, mon | 0.002 | ||
| ŌĆā< 1 | 7 (21.2) | 12 (30.0) | |
| ŌĆā1ŌĆō6 | 13 (39.4) | 5 (12.5) | |
| ŌĆā7ŌĆō12 | 10 (30.3) | 6 (15.0) | |
| ŌĆāŌēź 13 | 3 (9.1) | 17 (42.5) |
Values are presented as mean ┬▒ SD or number (%).
HIV, human immunodeficiency virus; HAART, highly active antiretroviral therapy; CDC, Centers for Disease Control and Prevention; PI, protease inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitors; ZDV, zidovudine; TMP/SMX, trimethoprim/sulfamethoxazole.
a According to the CDC classification [19].
b Defined as the surveillance case definitions for HIV infection and acquired immunodeficiency syndrome (AIDS) of CDC [19].
c According to the backbone antiretroviral drugs administered. The boosted PI-based regimen is defined as a ritonavir boosted PI-containing regimen. The mixed regimen is defined as switching from one class to another [19].



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



