 |
 |
| Korean J Intern Med > Volume 36(1); 2021 > Article |
|
Abstract
Background/Aims
Methods
Results
Acknowledgments
Supplementary Materials
Supplementary Table 1.
Supplementary Table 2.
Supplementary Table 3.
Supplementary Table 4.
Supplementary Figure 1.
Figure 1.
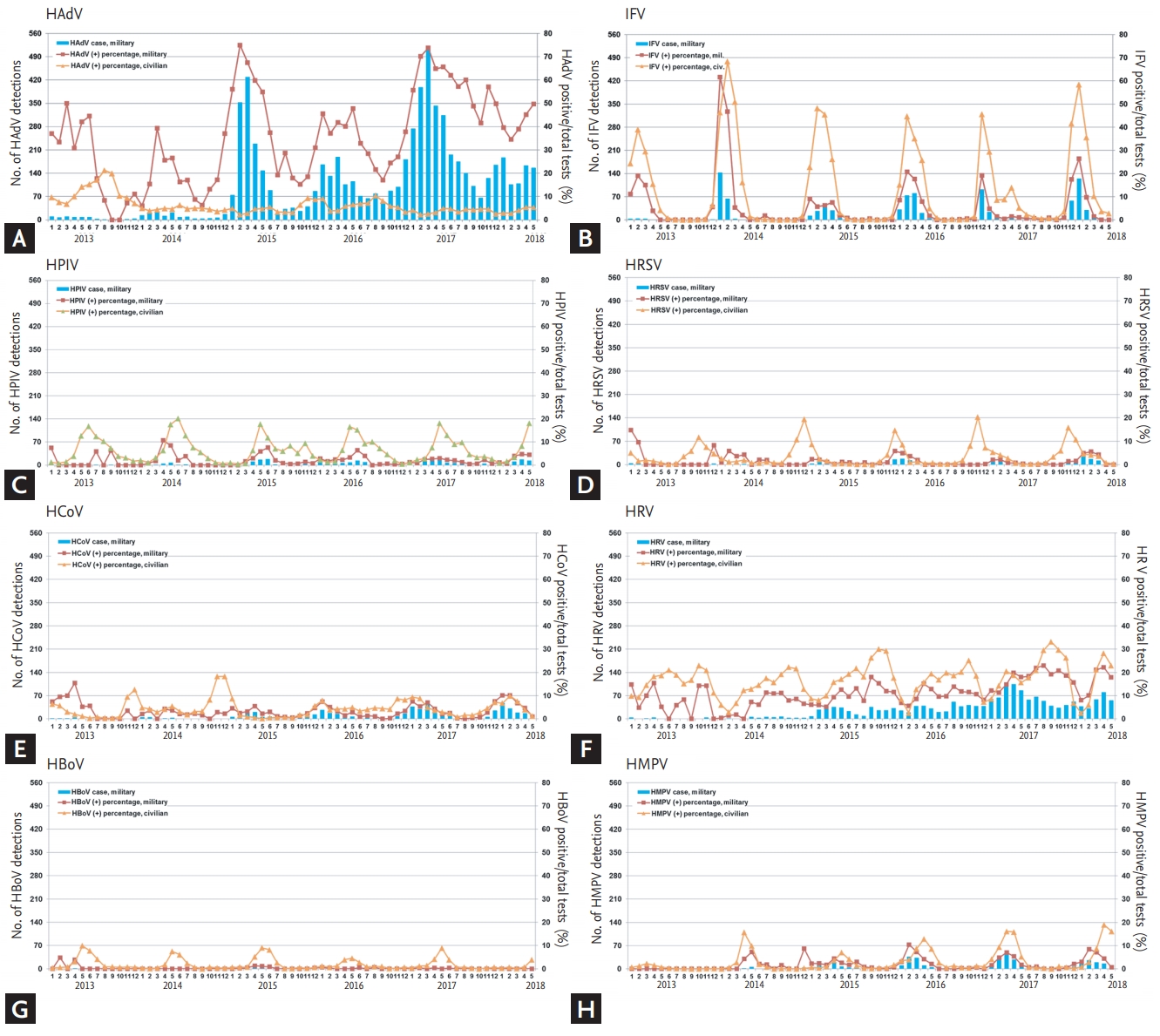
Figure 2.
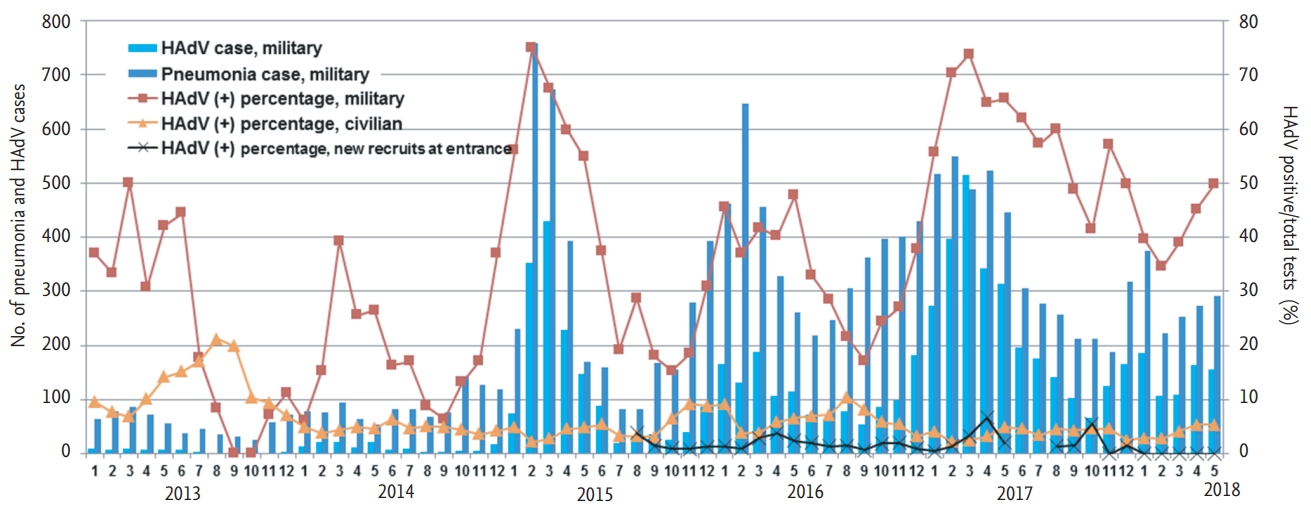
Figure 3.

Table 1.
| RVs |
January 2013–May 2013 |
June 2013–May 2014 |
June 2014–May 2015 |
June 2015–May 2016 |
June 2016–May 2017 |
June 2017–May 2018 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Military | Civilian | Military | Civilian | Military | Civilian | Military | Civilian | Military | Civilian | Military | Civilian | |
| HAdV | 43 (38.1)a,b | 686 (9.6)a,b | 109 (15.8)a,b | 1,274 (9.9)a,b | 1,286 (57.9)a,b | 477 (4.3)a,b | 1,037 (34.6)a,b | 636 (5.8)a,b | 2,480 (48.6)a,b | 592 (5.1)a,b | 1,691 (48.2)a,b | 485 (4.0)a,b |
| IFV | 11 (9.7)a | 1,662 (23.2)a | 213 (30.9)a,b | 2,092 (16.2)a,b | 114 (5.1)a | 1,574 (14.3)a | 213 (7.1)a | 1,353 (12.3)a | 144 (2.8)a | 1,197 (10.2)a | 225 (6.4)a | 2,029 (16.9)a |
| HPIV | 2 (1.8) | 265 (3.7) | 16 (2.3)a | 802 (6.2)a | 40 (1.8)a | 648 (5.9)a | 69 (2.3)a | 693 (6.3)a | 85 (1.7)a | 778 (6.6)a | 74 (2.1)a | 734 (6.1)a |
| HRSV | 6 (5.3)a,b | 161 (2.2)a,b | 18 (2.6) | 498 (3.9) | 25 (1.1)a | 485 (4.4)a | 62 (2.1)a | 315 (2.9)a | 34 (0.7)a | 505 (4.3)a | 61 (1.7)a | 565 (4.7)a |
| HCoV | 11 (9.7)a,b | 275 (3.8)a,b | 17 (2.5)a | 561 (4.4)a | 71 (3.2)a | 622 (5.7)a | 101 (3.4) | 361 (3.3) | 183 (3.6)a | 657 (5.6)a | 160 (4.6) | 549 (4.6) |
| HRV | 12 (10.6) | 983 (13.7) | 17 (2.5)a | 1,813 (14.1)a | 169 (7.6)a | 1,647 (15.0)a | 320 (10.7)a | 1,957 (17.8)a | 669 (13.1)a | 1,965 (16.8)a | 609 (17.4)a | 2,266 (18.9)a |
| HBoV | 2 (1.8) | 194 (2.7) | 0a | 229 (1.8)a | 12 (0.5)a | 248 (2.3)a | 6 (0.2)a | 204 (1.9)a | 7 (0.1)a | 239 (2.0)a | 1 (0.0)a | 113 (0.9)a |
| HMPV | 0 | 90 (1.3) | 8 (1.2) | 274 (2.1) | 56 (2.5)a,b | 155 (1.4)a,b | 108 (3.6) | 452 (4.1) | 124 (2.4)a | 668 (5.7)a | 91 (2.6)a | 530 (4.4)a |
| Positive, total | 73 (64.6) | 4,316 (60.3) | 376 (54.6)a | 7,542 (58.6)a | 1,539 (69.3)a,b | 5,851 (53.2)a,b | 1,656 (55.3) | 5,974 (54.4) | 3,202 (62.8)a,b | 6,599 (56.4)a,b | 2,498 (71.2)a,b | 7,270 (60.6)a,b |
| Test, total | 113 | 7,161 | 689 | 12,875 | 2,222 | 10,992 | 2,995 | 10,979 | 5,101 | 11,700 | 3,510 | 12,001 |
Values are presented as number (%). p values are presented in Supplementary Table 2.
RV, respiratory virus; HAdV, human adenovirus; IFV, influenza virus; HPIV, human parainfluenza virus; HRSV, human respiratory syncytial virus; HCoV, human coronavirus; HRV, human rhinovirus; HBoV, human bocavirus; HMPV, human metapneumovirus.
Table 2.
| Patient | Sex | Age, yr | Rank | MV, interval | Cidofovir, interval | ECMO, interval | Improvement of initial ARDS | Days of survival | Cause of death |
|---|---|---|---|---|---|---|---|---|---|
| Patient Aa | Male | 21 | Private | Yes, 5 dpoi | Unknown | Yes, unknown | No | 33 | Multi-organ failure |
| Patient B | Male | 20 | Corporal | Yes, 9 dpoi | Yes, 10 dpoi | No | No | 18 | Brain stem infarction |
| Patient Ca | Male | 23 | Staff sergeant | Yes, 6 dpoi | Unknown | Unknown | No | 12 | ARDS |
| Patient D | Male | 22 | Staff sergeant | Yes, 6 dpoi | Yes, 8 dpoi | Yes, 6 dpoi | Yes | 16 | Ventricular fibrillation |
| Patient E | Male | 20 | Private | Yes, 6 dpoi | Yes, 6 dpoi | Yes, 6 dpoi | Yes | 66 | Multi-organ failure |
HAdV, human adenovirus; ARI, acute respiratory illness; MV, mechanical ventilation; ECMO, extracorporeal membrane oxygenation; ARDS, acute respiratory distress syndrome; dpoi, days post onset of illness.
a Medical records of these patients were incomplete after referral to civilian hospitals. Although Patient A died due to multi-organ failure (MOF), the causal relationship with HAdV infection was not clear. Patient B died from brain death after brain stem infarction. Patient C died due to ARDS, most likely attributable to HAdV.
REFERENCES
- TOOLS



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print



