 |
 |
| Korean J Intern Med > Volume 35(2); 2020 > Article |
|
Abstract
Background/Aims
To compare the efficacy and safety of procedural sequence in same-day bidirectional endoscopy.
Methods
We searched OVID-MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, and Google Scholar to identify randomized controlled trials that compared the procedural sequences in same-day bidirectional endoscopy, including esophagogastroduodenoscopy (EGD) and colonoscopy. The sedative and analgesic doses required, discomfort and satisfaction scores, procedure time, recovery time, adenoma detection rate, and failed cecal intubation were evaluated. Adverse effects, including respiratory and cardiovascular complications, were also assessed.
Results
We included six studies, with 1,848 patients in total. The requirement for sedative treatment was significantly lesser in the EGD-colonoscopy sequence than in the colonoscopy-EGD sequence (standardized mean difference [SMD], –0.39; 95% confidence interval [CI], –0.54 to –0.24; p = 0.12; I2 = 49%). Discomfort, scored by patients during the EGD procedure, was significantly lesser in the EGD-colonoscopy sequence than in the colonoscopy-EGD sequence (SMD, –0.45; 95% CI, –0.80 to –0.09; p = 0.02; I2 = 73%), while it was comparable during colonoscopy between the two sequences. Recovery time was significantly shorter in the EGD-colonoscopy sequence than in the colonoscopy-EGD sequence (SMD, –0.47; 95% CI, –0.65 to –0.30; p = 0.28; I2 = 21%). Total procedure duration, EGD, colonoscopy, cecal intubation time and incidence, incidences of pathologic findings, and adenoma detection were comparable between the two sequences. There was no significant difference in the incidences of desaturation, hypotension, hypertension, bradycardia, and tachycardia between the two sequences.
Bidirectional endoscopy (BDE), a combination of esophagogastroduodenoscopy (EGD) and colonoscopy, is used to evaluate gastrointestinal conditions in patients with positive fecal occult blood tests, iron deficiency anemia, gastrointestinal bleeding, and abdominal pain [1]. BDE is also performed during a physical check-up or cancer screening. According to the national endoscopic database in the United States of America, more than 10% of patients who underwent upper or lower endoscopy had same-day BDE [1].
Performing both procedures on the same day is convenient for patients and reduces medical costs. Although the indications and benefits of same-day BDE are well-established, there is no clear consensus on the optimal procedural sequence. Indeed, several studies have compared the efficacy and safety of procedural sequences in same-day BDE, with conflicting results. Carter et al. [2] reported that the procedural sequence did not affect patients’ discomfort and satisfaction, and Choi et al. [3] presented that there was no significant difference in the colonoscopy performance and quality between upper and lower gastrointestinal endoscopy in same-day BDE. Conversely, some researchers demonstrated that for same-day BDE, EGD followed by colonoscopy was the optimal procedural sequence in terms of patients’ tolerance, recovery, or required sedative dose [4-8]. However, an anesthesiologist’s viewpoint suggested that colonoscopy followed by EGD was preferable [9] and asserted that when BDE is performed under deep sedation, EGD preceded by colonoscopy was more tolerable because the latter is usually performed under a deeper level of sedation [9].
Therefore, we aimed to perform a systematic review and meta-analysis to identify and summarize the evidence from randomized controlled trials comparing procedural sequences in same-day BDE in adult patients. The primary outcome was focused on efficacy, and the secondary outcome was focused on safety.
This systematic review and meta-analysis was registered in PROSPERO (CRD42019124390) and was conducted as per the protocol recommended by the Cochrane Collaboration [10] and following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11].
This systematic review and meta-analysis did not require ethics approval or informed consent because there was no direct contact with individual patients, and only previously published data were included in this study.
Two authors (G.J.C. and H.K.S.) independently searched the OVID-MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), and Google Scholar databases in October 2018. There were no language limitations to the search. The reference lists of the identified studies and eligible articles were also manually searched. The search strategy, which included a combination of free text, Medical Subject Headings, and EMTREE terms is described in the Appendix 1.
The study’s inclusion and exclusion criteria were determined before the systematic search. Randomized controlled trials that compared the effects of procedural sequences for BDE (EGD-colonoscopy vs. colonoscopy-EGD) were included. Review articles, case reports, cohort studies, case series, letters to the editor, commentaries, proceedings, laboratory science studies, and any other non-relevant studies were excluded. Two authors (G.J.C. and H.K.S.) independently scanned the titles and abstracts of the reports identified via the search strategies described above. If a report was deemed eligible from the title or abstract, the full text was retrieved. Potentially relevant studies selected by at least one author were retrieved, and full-text versions were evaluated. Two authors (G.J.C. and H.K.S.) discussed whether each study should be included. Disagreements over inclusions or exclusions were settled through discussions involving a third investigator (H.K.).
All interrelated data from the included studies were independently extracted and entered into standardized forms by two authors (H.C.O. and J.W.K.) and then cross-checked. Any discrepancy was resolved via a discussion. If an agreement could not be reached, the dispute was resolved with the aid of a third investigator (H.K.). The standardized form included the following items: (1) title, (2) name of the first author, (3) name of journal, (4) year of publication, (5) country, (6) sex, (7) age, (8) institution, (9) premedication drug used, (10) sedative used, (11) number of participants, (12) sedation implementer, (13) Institutional Review Board (IRB) approval, (14) clinical trial registration, (15) primary endpoint, (16) total amount of sedative used, (17) total amount of analgesic used, (18) satisfaction score, (19) pain or discomfort score during the procedure, (20) duration of total procedure, EGD and colonoscopy, (21) cecal intubation, (22) pathology found during procedure, (23) adenoma detection rate, (24) complications including hypoxia, hypotension, bradycardia, tachycardia, and hypertension, (25) post-procedural complications including pain, abdominal fullness, nausea, dizziness, sore throat, and cough, (26) recovery time, and (27) risk of bias.
The quality of the studies was independently assessed by two authors (G.J.C. and J.S.K.) using the revised Cochrane risk of bias tool for randomized trials (RoB 2.0) [13]. The risk of bias was evaluated by considering the following five potential sources of bias: (1) bias arising from the randomization process; (2) bias due to deviations from intended interventions; (3) bias due to missing outcome data; (4) bias in measurement of the outcome; (5) bias in selection of the reported results.
We evaluated an overall risk of bias according to these domain-level assessments. The methodology for each domain was graded as “low risk of bias,” “some concerns,” and “high risk of bias.” An overall rating for each study was determined accordingly.
We conducted this meta-analysis using Review Manager version 5.3 (The Cochrane Collaboration, Oxford, UK) and Comprehensive Meta-Analysis software version 2.0 (Biostat, Englewood, NJ, USA). Two authors (H.C.O. and J.S.K.) independently input all data into the software. The pooled risk ratio (RR), mean difference (MD), standardized mean difference (SMD), and their 95% confidence intervals (CIs) were calculated for each outcome.
We used the chi-square test for homogeneity and the I2 test for heterogeneity. A level of 10% significance (p < 0.1) for the chi-squared statistic or an I2 > 50% was considered to indicate considerable heterogeneity. A fixed-effects model was selected when the p value for the chi-square test was > 0.10 and the I2 value was < 50% [10,14]. In cases where the I2 value was > 50%, the random-effects model was used. Since the number of combined studies that displayed substantial heterogeneity was < 10, t statistics (Hartung-Knapp-Sidik-Jonkman method) were used instead of Z test in all random-effects analysis to lower the error rate [15].
We calculated the number needed to treat based on the absolute risk reduction as an estimate of the overall clinical impact of the intervention [16]. We carried out sensitivity analyses to evaluate the influence of a single study on the overall effect estimate by excluding one study at a time in case of heterogeneity > 50%. Publication bias was not assessed because the number of included studies was < 10 [10].
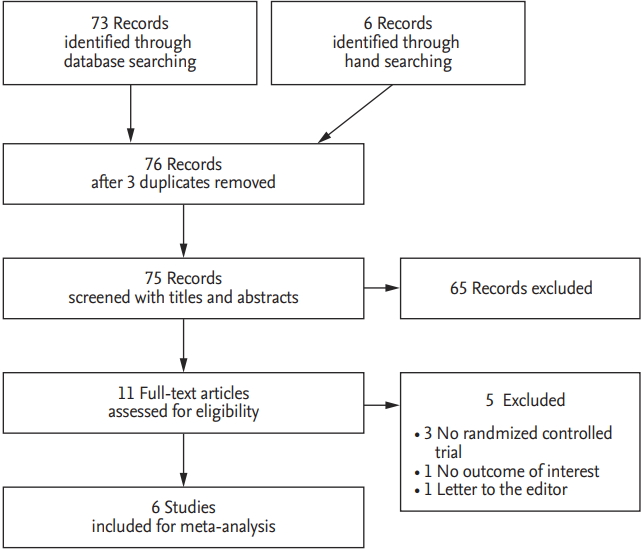
The search of OVID-MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) databases produced 73 studies, and six studies were identified by a manual research. After adjusting for duplicates, 76 studies remained. Of these, 65 studies were excluded because, after reviewing the title and abstracts, it appeared that these studies were not relevant to our review. The full texts of the remaining 11 studies were reviewed in detail, and five studies were excluded for the following reasons: not randomized controlled trials [1,8,17], no outcomes of our interest [18], and a letter to the editor [19]. Therefore, six studies with a total of 1848 patients met the inclusion criteria and were, therefore, included in this systematic review and meta-analysis (Fig. 1).
The studies were conducted in East Asia [3,4,6,7] and West Asia [2]. All the articles were written in English. A sedative was administered in four studies [2-4,7], of which a sedative was administered to a subset of participants in one study [3]. Sedation was performed by anesthesiologists in two studies [6,7] and, in one study, by an endoscopist and a nurse under the supervision of the endoscopist [5]. The study characteristics are summarized in Table 1.
Bias arising from the randomization process was assessed as a concern in four studies [2-4,6] and as a low risk in two studies [5,7]. The evaluations were low risk in all studies for the other bias domains [2-7] due to deviations from intended interventions, due to missing outcome date, in the measurement of the outcome, and selection of the reported results. The assessments of risk of bias are shown in Table 2.
The total amount of sedative used was reported in four studies [2,4,5,7]. The sedatives used were midazolam [2,5] and propofol [4,7]. The EGD-colonoscopy group showed a significant decrease in the total amount of sedative used compared with the colonoscopy-EGD group (SMD, –0.39; 95% CI, –0.54 to –0.24; p = 0.12; I2 = 49%) (Fig. 2). A subgroup analysis was conducted according to the type of sedative used. Midazolam and propofol were less required in the EGD-colonoscopy group than in the colonoscopy-EGD group ([midazolam: SMD, –0.53; 95% CI, –1.05 to –0.01; p = 0.03; I2 = 78%], [propofol: SMD, –0.32; 95% CI, –0.52 to –0.11; p = 0.99; I2 = 0%]). A sensitivity analysis was performed. When a study by Chen et al. was excluded, the value of I2 dropped to 0% (SMD, –0.30; 95% CI, –0.47 to –0.14; p = 0.98; I2 = 0%).
The total amount of analgesic used was similar between groups in two studies [2,4]. The analgesics used were meperidine [2], remifentanil [4], and fentanyl [5]. The combined results showed no evidence of a significant difference (SMD, –0.34; 95% CI, –0.77 to 0.10; p = 0.003; I2 = 82%). In terms of the analgesic dose used, a sensitivity analysis conducted showed that by removing one study at a time, the significance did not change.
Overall satisfaction scores evaluated by patients for both EGD and colonoscopy were reported in three studies [2,4,7]. These studies used a 10-point scale for evaluating satisfaction. The combined results showed no evidence of a significant difference between the two groups (SMD, –0.03; 95% CI, –0.20 to 0.14; p = 0.54; I2 = 0%). Two studies reported a preferential sequence [3,6]. The satisfaction scores of the endoscopist and anesthesiologist were reported in one study [4], which showed no significant difference between the EGD-colonoscopy and colonoscopy-EGD groups (8.60 ± 0.98 vs. 8.62 ± 0.99, p = 0.922; 8.59 ± 0.97 vs. 9.64 ± 0.98, p = 0.737, respectively). Patient satisfaction scores relating to sedation were reported in two studies [2,7]. The combined results showed no evidence of a significant difference (SMD, –0.01; 95% CI, –0.22 to 0.21; p = 0.49; I2 = 0%).
A discomfort score during the procedure was provided by patients in three studies [3,5,6]. The discomfort score provided by patients was significantly lower in the EGD-colonoscopy group during the EGD procedure than in the colonoscopy-EGD group (SMD, –0.45; –95% CI, –0.80 to –0.09; p = 0.02; I2 = 73%) (Fig. 3). There was no significant difference apparent during the colonoscopy procedure between the two groups (SMD, 0.00; –95% CI, –0.14 to 0.15; p = 0.59; I2 = 0%) (Fig. 3). The sensitivity analysis did not change the significance.
The discomfort score during the procedure was as Table 2. Assessment of the risk of bias based on the Cochrane risk of bias tool (RoB 2.0) Study sessed by endoscopists in three studies [2,5,7]. This score was expressed in various ways such as discomfort score [5], pain score [2], tolerance during EGD [7], or movement during colonoscopy [7]. These three studies used a 10-point evaluation scale. The discomfort score evaluated by the endoscopists during the EGD procedures showed no evidence of a significant difference between the two groups (SMD, –0.15; 95% CI, –0.50 to 0.19; p = 0.03; I2 = 71%). There was also no significant difference apparent during the colonoscopy procedure between the two groups (SMD, –0.27; 95% CI, –0.58 to 0.03; p = 0.07; I2 = 62%). The sensitivity analysis did not change the significance.
The durations of EGD and colonoscopy were reported in all studies. Duration for the total procedure was reported in three studies [2,3,5,7]. In the studies which did not report the total procedure duration [4,6], this was calculated by summing each EGD and colonoscopy duration. The combined results for the duration of EGD, colonoscopy, and total procedure showed no evidence of a difference in terms of EGD (SMD, –0.08; 95% CI, –0.17 to 0.01; p = 0.88; I2 = 0%) (Fig. 4); colonoscopy (SMD, –0.01; 95% CI, –0.10 to 0.08; p = 0.89; I2 = 0%) (Fig. 4); and total procedure (SMD, –0.01; 95% CI, –0.10 to 0.08; p = 0.94; I2 = 0%). The sensitivity analysis did not change the significance.
The cecal intubation time was reported in four studies [2,3,6,7]. The combined results showed no evidence of a difference (SMD, –0.03; 95% CI, –0.13 to 0.07; p = 0.91; I2 = 0.0%). Incidences of failure in cecal intubation were reported in three studies [3,5,7]. The combined results showed no evidence of a difference (risk ratio [RR], 1.27; 95% CI, 0.32 to 5.13; p = 0.26; I2 = 21%; number needed to treat harm [NNTH], 706.2; 95% CI, NNTH 113.0–∞ to number needed to treat benefit [NNTB] 166.2). The sensitivity analysis did not change the significance.
The incidences of pathologic findings during endoscopy were reported in four studies [2,4,6,7]. No significant difference with respect to pathology was found: EGD (RR, 1.06; 95% CI, 0.96 to 1.18; p = 0.93; I2 = 0%; NNTH, 33.8; 95% CI, NNTH 12.9 to ∞ to NNTB 14.5); and colonoscopy (RR, 0.94; 95% CI, 0.89 to 1.01; p = 0.03; I2 = 66%; NNTH, 47.7; 95% CI, NNTH 14.5–∞ to NNTB 36.7). Adenoma detection rates were reported in four studies [3- 5,7], which showed no significant difference between the two sequences (RR, 0.95; 95% CI, 0.68 to 1.33; p = 0.03; I2 = 67%) (Fig. 5) (NNTH, 75.5; 95% CI, NNTH 17.6–∞ to NNTB 32.9). The sensitivity analysis did not change the significance.
Hypoxia and hypotension in four studies [2,4,5,7] and bradycardia, tachycardia, and hypertension in three studies were reported [2,4,7]. There was no significant difference between the two sequences in terms of hypoxia (RR, 1.07; 95% CI, 0.34 to 3.38; p = 0.548; I2 = 0.0%; NNTH, 182.3; 95% CI, NNTH 36.8–∞ to NNTB 61.7); hypotension (RR, 1.48; 95% CI, 0.81 to 2.73; p = 0.991; I2 = 0.0%; NNTH, 49.6; 95% CI, NNTH 13.2–∞ to NNTB 28.4); bradycardia (RR, 1.31; 95% CI, 0.29 to 5.88; p = 0.945; I2 = 0.0%; NNTH, 227.0; 95% CI, NNTH 237.5–∞ to NNTB 76.8); tachycardia (RR, 0.97; 95% CI, 0.01 to 152.7; p = 1.000; I2 = 0.0%; NNTH, 37.6; 95% CI, NNTH 6,507.9–∞ to NNTB 18.7); and hypertension (RR, 0.30; 95% CI, 0.06 to 1.44; p = 0.828; I2 = 0.0%; NNTH 56.8; 95% CI, NNTH 28.8–∞ to NNTB 1,979.2). The sensitivity analysis did not change the significance.
The degree of pain, abdominal fullness, nausea, dizziness, sore throat and cough after the procedure was reported in two studies [2,7]. The combined results showed no evidence of a difference between the two sequences for the degree of pain (SMD, 0.11; 95% CI, –0.45 to 0.68; p = 0.009; I2 = 86%); abdominal fullness (SMD, 0.15; 95% CI, –0.07 to 0.36; p = 0.539; I2 = 0%); nausea (SMD, 0.00; 95% CI, –0.77 to 0.77; p < 0.001; I2 = 92%); dizziness (SMD, –0.04; 95% CI, –0.50 to 0.42; p = 0.032; I2 = 78%); sore throat (SMD, –0.20; 95% CI, –0.41 to 0.02; p = 0.70; I2 = 0%); and cough (SMD, –0.06; 95% CI, –0.28 to 0.15; p = 0.55; I2 = 0%).
The incidences of nausea, cough, and abdominal fullness after the procedure were reported in one study [4]. There was no significant difference between the EGD-colonoscopy and colonoscopy-EGD sequence regarding nausea, 0 vs. 1; cough, 0 vs. 2; and abdominal fullness, 1 vs. 0. The sensitivity analysis did not change the significance.
The recovery time was reported in three studies [4,5,7]. Hsieh et al. [7] reported a recovery time at the following two different intervals: time to talking and time to discharge. The recovery time was significantly shorter in the EGD-colonoscopy group than in the colonoscopy-EGD group for both time intervals, i.e., time to talking and time to discharge, respectively (SMD, –0.47; 95% CI, –0.65 to –0.30; p = 0.28; I2 = 21%) (Fig. 6) (SMD, –0.40; 95% CI, –0.77 to –0.02; p = 0.01; I2 = 77%). The sensitivity analysis did not change the significance.
In the present study, patients required a significantly lower dose of sedative when EGD preceded colonoscopy. This suggests that there are several advantages when EGD precedes colonoscopy in same-day BDE. These advantages include a lower dose of sedative used and a low incidence of complications, such as delayed recovery or desaturation, caused by high doses of sedatives. Sedative overdose is a crucial issue in procedural sedation, and a lot of effort has been put in clinical practice to optimize the sedative dose without causing serious complications [20]. This can be supported by the results in this study, which showed that the recovery time was significantly shorter when EGD preceded colonoscopy. A shorter recovery time is linked to a quick return to the daily life of patients as well as a faster rate of hospital bed turnover.
Furthermore, smaller doses of sedatives used can result in lower medical costs. Several studies investigated the cost-effectiveness of sedation during procedural sedation and suggested that a decrease in sedative cost can reduce medical expenses [18,21]. A lower sedative dose can potentially result in fewer complications and a shorter recovery time, which in turn results in lower health care costs. The studies evaluated in this current meta-analysis used different types of sedatives, with some studies using analgesics for pain control [2,4,5]. We conducted a subgroup analysis according to the types of sedatives and the use of analgesics. However, the significance or heterogeneity of the results did not change.
Differences in sedative dosage may result from different procedure characteristics. For example, EGD usually requires more sedation when inserting the endoscope than a colonoscopy, and the duration of EGD is shorter than that of colonoscopy. Hence, in EGD followed by colonoscopy, the sedation achieved during EGD can result in a lesser dose of sedative being required during the colonoscopy. Our results showed that patients required a significantly lower dose of sedative when EGD preceded colonoscopy.
Despite the many benefits of using a lower sedative dose in BDE, there is a possibility that patient satisfaction for the procedure or sedation will suffer. The sedative dose required to prevent the side effects mentioned may be insufficient to maintain satisfactory procedures or sedation for patients and clinicians. However, sedative overdose did not seem to result in a reduction in patient satisfaction in this study. The satisfaction scores of patients, endoscopists, and anesthesiologists for the procedure and sedation were comparable between the two sequences.
The incidences of complications, such as desaturation, hypotension, and bradycardia, were also similar. Of five studies conducted on procedural sedation [2-5,7], the depth of sedation was targeted at a deep level in three studies [3,4,7] and a moderate level in one study [5]. Given that deep sedation puts the patient at an increased risk for respiratory and cardiovascular depression, the colonoscopy-EGD sequence was not inferior to the EGD-colonoscopy sequence, at least in terms of safety.
The discomfort scored by patients during the procedure was not consistent with the results from the satisfaction scored by them. In the present study, the patients experienced a significantly greater measure of discomfort during the EGD procedure when EGD was preceded by colonoscopy, while discomfort scores during colonoscopy were comparable between the two sequences. Abdominal bloating induced by the colonoscopy procedure can make it difficult for patients to endure a subsequent EGD. Of the three studies reporting a discomfort score by patients [3,5,6], procedural sedation was not done in two studies [3,6], and moderate sedation was performed in one study [5]. It is of no surprise that patients evaluated their discomfort during an endoscopy procedure. The difference in the discomfort scores between the two groups during EGD may be reduced by using deep sedation. Our results support that the discomfort scores by endoscopists were comparable during both endoscopic procedures. Sedation targeted at a moderate to deep level was performed in all studies in which endoscopists reported a discomfort score.
All studies included in this meta-analysis were performed in Asia. We performed additional analyses, including Kurien et al.’s [19] trial performed in the United Kingdom on the Western population. Although we finally excluded this study in this meta-analysis because it was a letter to the editor, we conducted a different meta-analysis including Kurien et al.’s [19] study to reduce any bias in terms of race or region. We could extract only the discomfort score provided by patients, and there was no change in the results that patients experienced more discomfort during EGD when it was preceded by colonoscopy. Rather, the heterogeneity showed a great decrease (SMD, –1.47; 95% CI, –2.67 to –0.27; p = 0.12; I2 = 0%).
There were several limitations to this study. Firstly, the physicians who performed BDE and sedation were not blinded to the procedural sequence, which was inevitable considering the study design. Secondly, we need to consider that our results do not fully reflect actual clinical practice in a gastrointestinal endoscopic suite. Although we compared the total dose of sedative in this study, different sedatives may be used for each procedure in practice. That is, the sedative can mainly be propofol for EGD or midazolam for colonoscopy. In this case, the sedative dose may vary depending on the sequence of the sedative administered. In some cases, patients may have factors such as a medical history that may affect the sequence regardless of the benefits from the sequence itself. EGD followed by colonoscopy may be a better option in patients with highly suspected gastric cancer, whereas colonoscopy followed by EGD may be more favorable in patients with a history of abdominal surgery. Thirdly, some outcomes were heterogeneous. How sedation was performed, the use of analgesic, the sedation regimen, the sedation depth, the evaluation scale for satisfaction, and the discomfort or gas insufflation during the procedure varied, resulting in heterogeneous outcomes. Therefore, we conducted meticulous subgroup and sensitivity analyses. Finally, this meta-analysis included only a small number of studies.
In conclusion, when conducting same-day BDE, EGD followed by colonoscopy is the beneficial sequence because only a low sedative dose is required and the patient recovery time is faster with less discomfort. Complications relating to safety issues were similar regardless of the procedural sequence used.
1. Same-day bidirectional endoscopy is convenient for patients and can reduce medical costs.
2. This study provides evidence of the beneficial sequence and examines any safety issues. Esophagogastroduodenoscopy, followed by colonoscopy, can be considered as a superior procedural sequence in terms of faster recovery time, lower sedative dose, and reduced patient discomfort. Further, less heterogeneous evidence is necessary to draw a definite conclusion.
3. The study will provide useful and novel information for patients, physician, and policymakers.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2018R1A2A2A05021467).
Figure 2.
Forest plot of the sedative dose used. EDG, esophagogastroduodenoscopy; SD, standard deviation; IV, inverse variance;
CI, confidence interval.

Figure 3.
Forest plot of patient discomfort scores. EDG, esophagogastroduodenoscopy; SD, standard deviation; IV, inverse variance; CI, confidence interval.

Figure 4.
Forest plot of procedural duration. EDG, esophagogastroduodenoscopy; SD, standard deviation; IV, inverse variance; CI, confidence interval.

Figure 5.
Forest plot of adenoma detection rate. EDG, esophagogastroduodenoscopy; M-H, Mantel-Haenszel; CI, confidence interval.
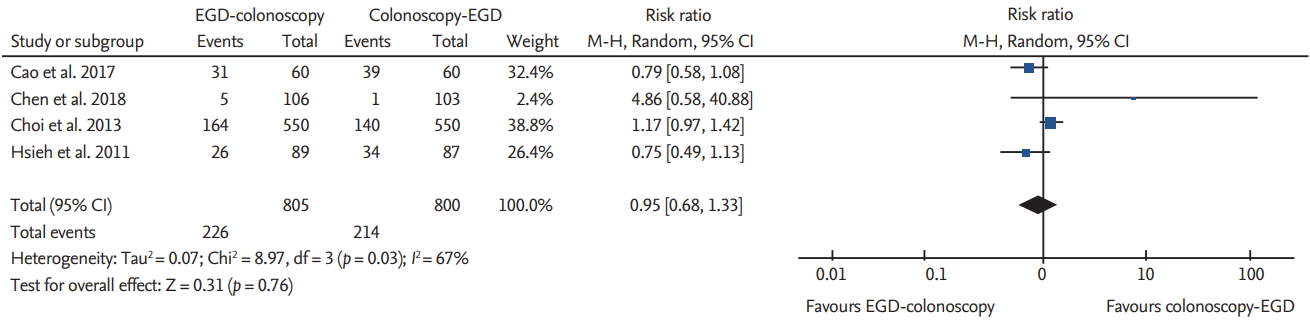
Figure 6.
Forest plot of recovery time. EDG, esophagogastroduodenoscopy; SD, standard deviation; IV, inverse variance; CI, confidence interval.
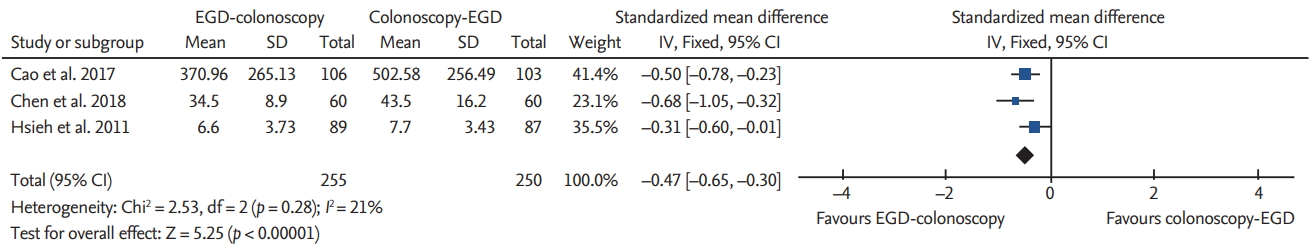
Table 1.
Study characteristics
| Study | Country | No. (E-C/C-E) | Sedation regimen (initial dose→additional dose) | Sedation depth | Analgesic used | Clinical trial registry | IRB |
|---|---|---|---|---|---|---|---|
| Chen et al. (2018) [5] | Taiwan | 60/60 | Midazolam 1.5 mg → 0.5–1.0 mg | Moderate | Fentanyl 50 μg→10–20 μg | NCT | A |
| 2708212 | |||||||
| Cao et al. (2017) [4] | China | 106/103 | Propofol 2 mg/kg → 0.5 mg/kg | Deep | Remifentanil 1 μg/kg | ChiCTR-IOR | A |
| -16007946 | |||||||
| Carter et al. (2014) [2] | Israel | 83/80 | Midazolam 2.5 mg → 1.25–2.5 mg | NR | Meperidine 50 mg | NCT | A |
| 1491126 | |||||||
| Choi et al. (2013) [3] | Korea | 550/550 | Midazolam 3–5 mga → Propofol 10–20 mg | Deep | Meperidine 25 mg | KCT | A |
| 430 | |||||||
| Hsieh et al. (2011) [7] | Taiwan | 89/87 | Propofol 1 mg/kg → 10–20 mg | Deep | Meperidine 25 mg | NR | A |
| Cho et al. (2010) [6] | Korea | 40/40 | NA | NA | NA | NR | A |
E-C, esophagogastroduodenoscopy (EGD)-colonoscopy sequence; C-E, colonoscopy-EGD sequence; IRB, Institutional Review Board; NCT, United States clinical trial registry; A, approved; ChiCTR, Chinese clinical trial registry; IOR, interquartile range; NR, not reported; KCT, Korean clinical trial registry; NA, not applicable.; NR, not registrered.
Table 2.
Assessment of the risk of bias based on the Cochrane risk of bias tool (RoB 2.0)
| Study | Bias arising from the randomization process | Bias due to deviations from intended interventions | Bias due to missing outcome data | Bias in measurement of the outcome | Bias in the selection of the reported results | Overall bias |
|---|---|---|---|---|---|---|
| Chen et al. (2018) [5] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Cao et al. (2017) [4] | Some concerns | Low risk | Low risk | Low risk | Low risk | Some concerns |
| Carter et al. (2014) [2] | Some concerns | Low risk | Low risk | Low risk | Low risk | Some concerns |
| Choi et al. (2013) [3] | Some concerns | Low risk | Low risk | Low risk | Low risk | Some concerns |
| Hsieh et al. (2011) [7] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Cho et al. (2010) [6] | Some concerns | Low risk | Low risk | Low risk | Low risk | Some concerns |
REFERENCES
1. Urquhart J, Eisen G, Faigel DO, Mattek N, Holub J, Lieberman DA. A closer look at same-day bidirectional endoscopy. Gastrointest Endosc 2009;69:271–277.


2. Carter D, Lahat A, Papageorgiou NP, Goldstein S, Eliakim R, Bardan E. Comparison of procedural sequence in same-day consecutive bidirectional endoscopy using moderate sedation: a prospective randomized study. J Clin Gastroenterol 2014;48:236–240.


3. Choi JS, Youn YH, Lee SK, et al. Which should go first during same-day upper and lower gastrointestinal endoscopy?: a randomized prospective study focusing on colonoscopy performance. Surg Endosc 2013;27:2209–2215.



4. Cao Y, Yang J, Li J, et al. Comparison of procedural sequences in same-day painless bidirectional endoscopy: single-center, prospective, randomized study. Dig Endosc 2017;29:330–337.


5. Chen SW, Cheng CL, Liu NJ, et al. Optimal procedural sequence for same-day bidirectional endoscopy with moderate sedation: a prospective randomized study. J Gastroenterol Hepatol 2018;33:689–695.


6. Cho JH, Kim JH, Lee YC, Song SY, Lee SK. Comparison of procedural sequences in same-day bidirectional endoscopy without benzodiazepine and propofol sedation: starting at the bottom or the top. J Gastroenterol Hepatol 2010;25:899–904.


7. Hsieh YH, Lin HJ, Tseng KC. Which should go first during same-day bidirectional endosocopy with propofol sedation? J Gastroenterol Hepatol 2011;26:1559–1564.


8. Tang JH, Cheng CL, Kuo YL, Tsui YN. Paired comparison of procedural sequence in same-day bidirectional endoscopy with moderate sedation and carbon dioxide insufflation: a prospective observational study. Saudi J Gastroenterol 2016;22:360–365.



9. Lin JA. Procedural sequence of bidirectional gastrointestinal endoscopy from the anaesthesiologist’s viewpoint. Br J Anaesth 2014;113(eLetters Suppl):el11898.

10. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Chichester (UK): Cochrane Collaboration, 2011.
11. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1.


12. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13.




13. Higgins JPT, Sterne JAC, Savovic J, et al. A revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst Rev 2016;10(Suppl 1):29–31.
14. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560.



15. IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol 2014;14:25.




16. Naing C, Aung K, Mak JW. Reporting ‘number needed to treat’ in meta-analyses: a cross-sectional study. J Evid Based Med 2012;5:232–237.


17. Oner OZ, Demirci RK, Gunduz UR, Aslaner A, Koc U, Bulbuller N. Prior esophagogastroduodenoscopy does not affect the cecal intubation time at bidirectional endoscopies. Int J Clin Exp Med 2013;6:599–602.


18. Lucendo AJ, Arias A, Gonzalez-Castillo S, et al. Sameday bidirectional endoscopy with nonanesthesiologist administration of propofol: safety and cost-effectiveness compared with separated exams. Eur J Gastroenterol Hepatol 2014;26:301–308.


19. Kurien M, Din S, Dear KL, Elphick DA. Same day bidirectional endoscopy: does the procedural order matter? J Gastrointestin Liver Dis 2012;21:328.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



