 |
 |
| Korean J Intern Med > Volume 35(5); 2020 > Article |
|
Abstract
Background/Aims
Methods
Results
Acknowledgments
Supplementary Materials
Supplementary Figure 1.
Figure 1.

Figure 2.

Figure 3.
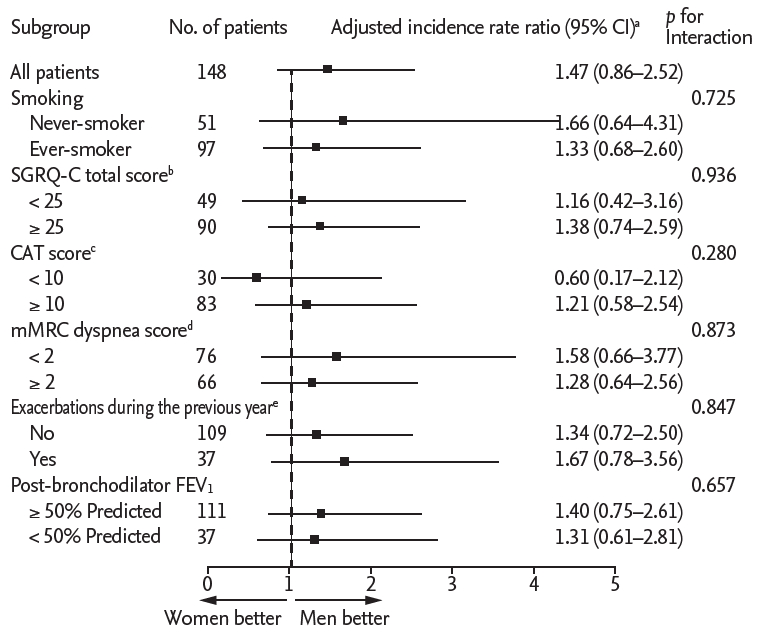
Figure 4.
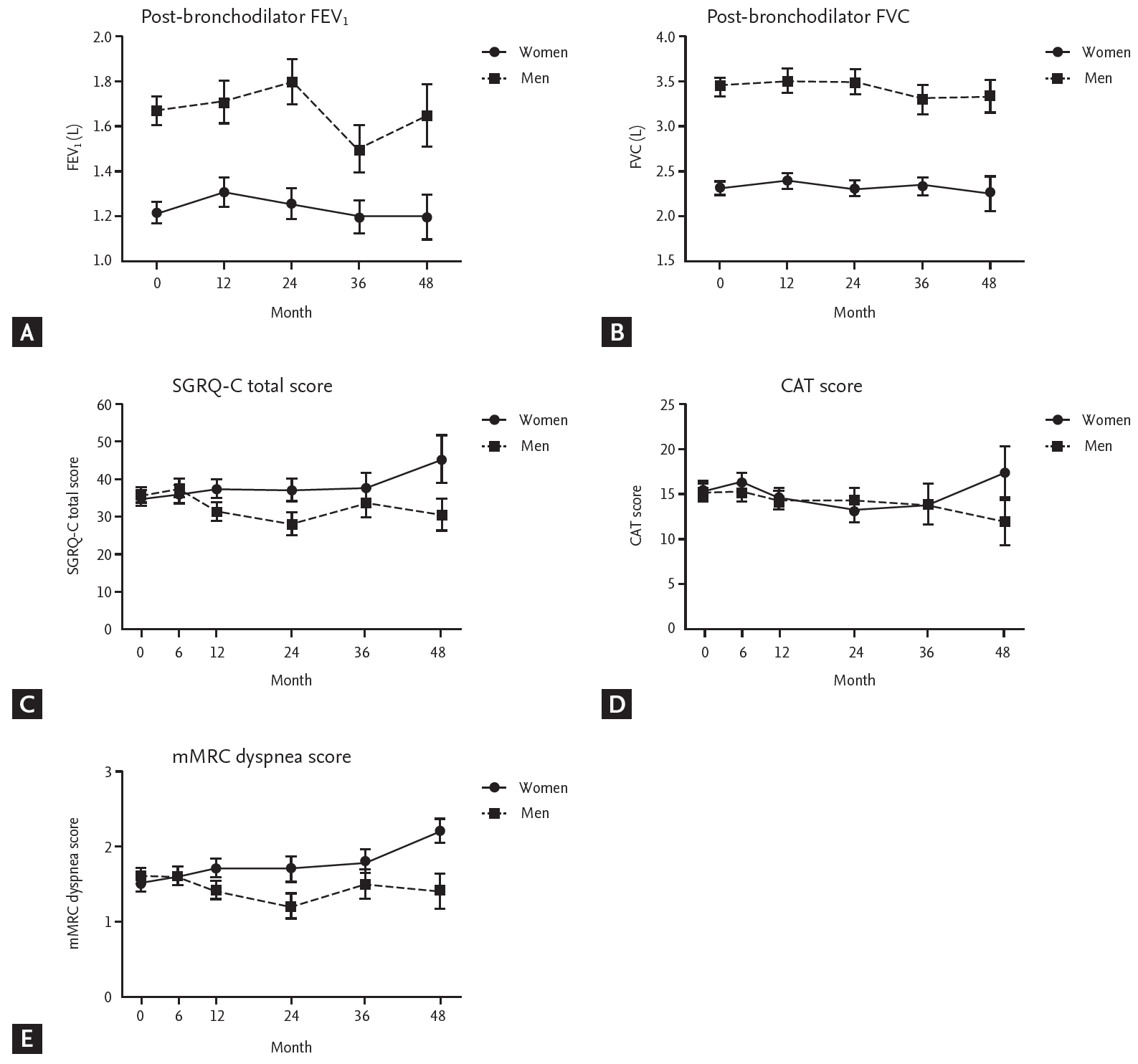
Table 1.
| Characteristic |
Before propensity score matching |
After propensity score matching |
||||
|---|---|---|---|---|---|---|
| Men (n = 1,315) | Women (n = 89) | p value | Men (n = 74) | Women (n = 74) | p value | |
| Age, yr | 68.5 ± 7.5 | 69.2 ± 7.4 | 0.394 | 68.3 ± 8.1 | 68.6 ± 7.6 | 0.794 |
| BMI, kg/m2 | 22.9 ± 3.3 | 23.7 ± 3.7 | 0.016 | 24.0 ± 3.2 | 23.5 ± 3.7 | 0.334 |
| Smoking | < 0.001 | 0.268 | ||||
| Never-smoker | 22 (1.7) | 44 (49.4) | 22 (29.7) | 29 (39.2) | ||
| Former smoker | 921 (70.0) | 24 (27.0) | 22 (29.7) | 24 (32.4) | ||
| Current s moker | 372 (28.3) | 21 (23.6) | 30 (40.5) | 21 (28.4) | ||
| Smoking, pack-years | 44.7 ± 25.8 | 15.7 ± 20.9 | < 0.001 | 20.7 ± 21.0 | 18.9 ± 21.6 | 0.615 |
| Exposure to biomass fuel, yr | < 0.001 | 0.410 | ||||
| < 10 | 423 (32.2) | 9 (10.1) | 12 (16.2) | 9 (12.2) | ||
| ≥ 10 | 539 (41.0) | 62 (69.7) | 39 (52.7) | 47 (63.5) | ||
| Unknown | 353 (26.8) | 18 (20.2) | 23 (31.1) | 18 (24.3) | ||
| Exacerbations during the previous year | 0.528 | > 0.999 | ||||
| No | 954 (72.6) | 64 (71.9) | 55 (74.3) | 54 (73.0) | ||
| Yes | 321 (24.4) | 24 (27.0) | 18 (24.3) | 19 (25.7) | ||
| Unknown | 40 (3.0) | 1 (1.1) | 1 (1.4) | 1 (1.4) | ||
| SGRQ-C total scorea | 34.7 ± 19.0 | 37.5 ± 19.8 | 0.188 | 35.6 ± 20.3 | 35.1 ± 18.4 | 0.892 |
| SGRQ-C total score | 0.525 | 0.929 | ||||
| < 25 | 462 (35.1) | 26 (29.2) | 25 (33.8) | 24 (32.4) | ||
| ≥ 25 | 798 (60.7) | 59 (66.3) | 44 (59.5) | 46 (62.2) | ||
| Unknown | 55 (4.2) | 4 (4.5) | 5 (6.8) | 4 (5.4) | ||
| CAT scoreb | 15.1 ± 8.0 | 16.3 ± 8.9 | 0.210 | 15.6 ± 8.6 | 15.3 ± 7.8 | 0.833 |
| CAT score | 0.827 | 0.874 | ||||
| < 10 | 279 (21.2) | 20 (22.5) | 14 (18.9) | 16 (21.6) | ||
| ≥ 10 | 767 (58.3) | 49 (55.1) | 43 (58.1) | 40 (54.1) | ||
| Unknown | 269 (20.5) | 89 (22.5) | 17 (23.0) | 18 (24.3) | ||
| mMRC dyspnea scorec | 1.5 ± 1.0 | 1.6 ± 0.9 | 0.494 | 1.6 ± 1.0 | 1.5 ± 0.9 | 0.856 |
| mMRC dyspnea score | 0.726 | > 0.999 | ||||
| < 2 | 706 (53.7) | 44 (49.4) | 38 (51.4) | 38 (51.4) | ||
| ≥ 2 | 564 (42.9) | 42 (47.2) | 33 (44.6) | 33 (44.6) | ||
| Unknown | 45 (3.4) | 3 (3.4) | 3 (4.1) | 3 (4.1) | ||
| Post-bronchodilator FEV1, % predicted | 58.8 ± 17.9 | 65.8 ± 20.7 | 0.002 | 60.8 ± 19.0 | 61.9 ± 16.9 | 0.700 |
| Post-bronchodilator FVC, % predicted | 85.6 ± 18.0 | 89.4 ± 18.5 | 0.054 | 87.5 ± 19.9 | 86.6 ± 17.2 | 0.773 |
| Post-bronchodilator FEV1/FVC, % | 48.4 ± 11.4 | 53.6 ± 10.9 | < 0.001 | 49.0 ± 11.8 | 52.6 ± 10.7 | 0.053 |
| Bronchodilator response (FEV1), % | 7.9 ± 11.8 | 7.1 ± 13.6 | 0.618 | 7.0 ± 10.5 | 7.7 ± 14.0 | 0.749 |
| Blood eosinophil, /μLd | 266 ± 314 | 206 ± 208 | 0.022 | 208 ± 192 | 225 ± 224 | 0.671 |
| Blood eosinophil, %e | 3.6 ± 3.7 | 2.8 ± 2.5 | 0.018 | 2.8 ± 2.5 | 3.1 ± 2.7 | 0.564 |
| Blood eosinophil | 0.133 | 0.663 | ||||
| ≤ 5% | 842 (64.0) | 66 (74.2) | 50 (67.6) | 52 (70.3) | ||
| > 5% | 217 (16.5) | 9 (10.1) | 7 (9.5) | 9 (12.2) | ||
| Unknown | 256 (19.5) | 14 (15.7) | 17 (23.0) | 13 (17.6) | ||
| Use of LAMA at enrollment | 701 (53.3) | 35 (39.3) | 0.011 | 32 (43.2) | 32 (43.2) | > 0.999 |
| Use of LABA at enrollment | 702 (53.4) | 46 (51.7) | 0.756 | 49 (66.2) | 38 (51.4) | 0.066 |
| Use of ICS at enrollment | 554 (42.1) | 42 (47.2) | 0.350 | 42 (56.8) | 34 (46.0) | 0.188 |
Values are presented as mean ± SD or number (%).
BMI, body mass index; SGRQ-C, St. George’s Respiratory Questionnaire for COPD; CAT, COPD assessment test; COPD, chronic obstructive pulmonary disease; mMRC, modified Medical Research Council; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; LAMA, long-acting muscarinic antagonist; LABA, long-acting β-agonist; ICS, inhaled corticosteroid.
a Data are for 1,260 men and 85 women before propensity score matching, and 69 men and 70 women after propensity score matching.
b Data are for 1,046 men and 69 women before propensity score matching, and 57 men and 56 women after propensity score matching.
c Data are for 1,270 men and 86 women before propensity score matching, and 71 men and 71 women after propensity score matching.
Table 2.
| Variable |
Before propensity score matching |
After propensity score matching |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Men (n = 1,315) |
Women (n = 89) |
IRRa (95% CI) | IRRb (95% CI) |
Men (n = 74) |
Women (n = 74) |
IRRa (95% CI) | IRRc (95% CI) | |||||
| No. (%) | IRa | No. (%) | IRa | No. (%) | IRa | No. (%) | IRa | |||||
| Exacerbations | 574 (43.7) | 0.65 | 38 (42.7) | 0.62 | 0.95 (0.66–1.38) | 1.12 (0.74–1.71) | 32 (43.2) | 0.44 | 33 (44.6) | 0.66 | 1.49 (0.88–2.54) | 1.47 (0.86–2.52) |
b Adjusted for age, body mass index, smoking status (never-smoker vs. former smoker vs. current smoker), exacerbation history during the previous year (yes vs. no vs. unknown), St. George’s Respiratory Questionnaire for chronic obstructive pulmonary disease (COPD) total score (< 25 vs. ≥ 25), and % predicted post-bronchodilator FEV1 (as continuous variables).
REFERENCES
- TOOLS
-
METRICS

- Related articles
-
High prevalence of chronic obstructive pulmonary disease in Korea2016 July;31(4)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print


