 |
 |
| Korean J Intern Med > Volume 36(2); 2021 > Article |
|
Abstract
Background/Aims
The aim of this study was to compare antimicrobial resistance, clinical features, and outcomes of community-onset Escherichia coli (COEC) and Klebsiella pneumoniae (COKP) bacteremia.
Methods
The medical records of patients diagnosed with E. coli or K. pneumoniae bacteremia in the emergency department of a 750-bed secondary care hospital in Daegu, Korea from January 2010 to December 2016 were retrospectively reviewed.
Results
A total of 866 patients with COEC bacteremia and 299 with COKP bacteremia were enrolled. COEC bacteremia, compared to COKP bacteremia, had higher rates of 3rd generation cephalosporin (3GC) (18.8% vs. 8.4%, p < 0.001) and f luoroquinolone (FQ) (30.4% vs. 8.0%, p < 0.001) resistance. The patients with COKP bacteremia had higher Charlson comorbidity indices (CCI) (1.8 ± 2.0 vs. 1.5 ± 1.8, p = 0.035), Pittsburgh bacteremia scores (PBS) (2.0 ± 2.6 vs. 1.3 ± 1.8, p < 0.001), and 30-day mortality (14.44% vs. 8.8%, p = 0.008) than the patients with COEC bacteremia. Age younger than 70 years, male sex, polymicrobial infections, pneumonia, intra-abdominal infection, PBS ≥ 2, and Foley catheter insertion were independent predictive factors for COKP bacteremia compared to COEC bacteremia in the multivariate analysis. CCI, PBS, and intensive care unit admission were independent risk factors for 30-day mortality in the multivariate analysis.
Our previous research, performed at a secondary care community-based hospital in Daegu, Korea between 2003 to 2009, showed that Escherichia coli and Klebsiella pneumoniae are major pathogens in community-onset bacteremia and that their levels of antimicrobial resistances are increasing, even in the community setting [1]. In that study, 3rd generation cephalosporin (3GC) and fluoroquinolone (FQ) resistance rates were 6.6% and 24.5% in E. coli and 4.9% and 4.9% in K. pneumoniae, respectively. A study of community-onset bacteremia in a tertiary hospital in Seoul, Korea between 2012 to 2015 showed that 27.2% of E. coli and 14.9% of Klebsiella species were extended spectrum β-lactam (ESBL) producers and 37.8% of E. coli and 21.0% of Klebsiella species had ciprofloxacin resistance [2]. The resistant rates to 3GC and FQ antimicrobials in Klebsiella species were consistently lower than in E. coli in both studies even though a recent study in a tertiary care hospital showed higher resistant rates. We sought to follow-up on our previous study of trend in antimicrobial resistance compared to the recent study conducted in a tertiary care hospital.
Although E. coli and K. pneumoniae bacteremia have been studied individually or together, studies comparing antimicrobial resistance and clinical features and outcomes in these two major pathogens have rarely been conducted in a secondary care community-based hospital [3,4]. The aims of this study were to analyze current trends in antimicrobial resistance and compare antimicrobial resistance and clinical features and outcomes in community-onset E. coli (COEC) and K. pneumoniae (COKP) bacteremia.
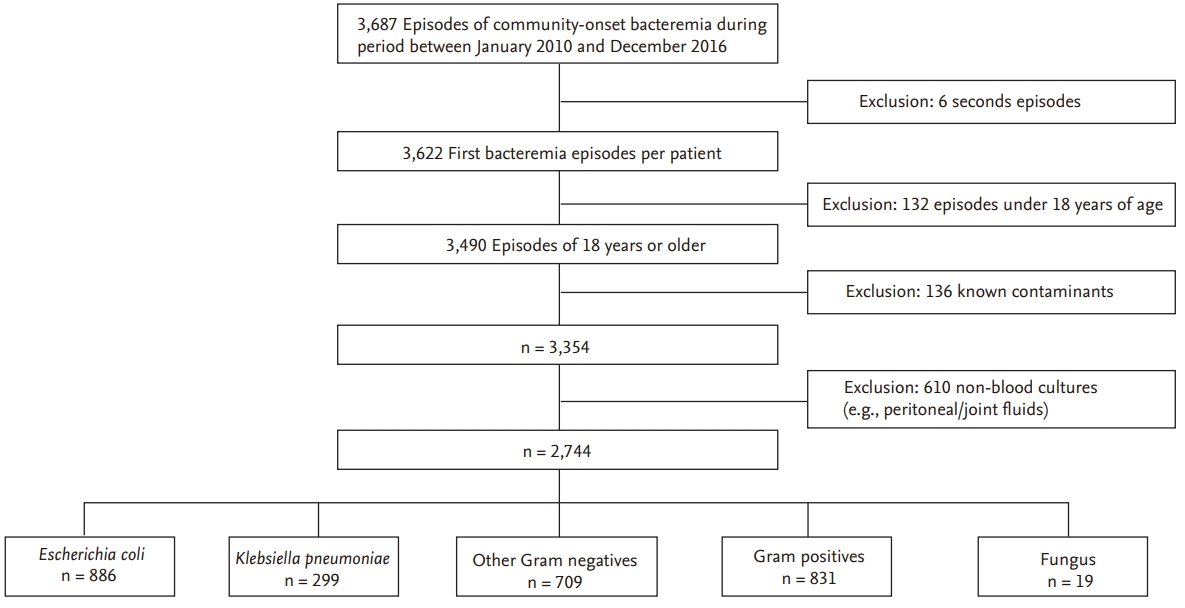
This retrospective cohort study was performed at Daegu Fatima Hospital, a 750-bed secondary care community-based hospital in Daegu, Korea. Patients presenting to the emergency department with a first episode of E. coli or K. pneumoniae bacteremia between January 2010 and December 2016 were enrolled and defined as community-onset bacteremia, and their clinical microbiology laboratory data were reviewed (Fig. 1). From the electronic medical records, we collected data on pathogen antimicrobial resistance rates, patient demographic characteristics, source of infection, severity of bacteremia (Pittsburgh bacteremia score [PBS]) [5], underlying diseases (Charlson comorbidity index [CCI]) [6], antimicrobial use, and outcomes. Sites of infection were defined according to the criteria of the Centers for Disease Control and Prevention. Antimicrobial therapy was considered to be appropriate if antimicrobial agents to which the pathogen was susceptible were administered within 48 hours after the onset of bacteremia. 3GC resistance was defined as intermediate or resistant to ceftazidime and/or cefotaxime. Fluoroquinolone resistance was defined as intermediate or resistant to ciprofloxacin and/or levofloxacin. This study was approved by the Institutional Review Board of the Daegu Fatima Hospital (DFH18ORIO354). Written informed consent by the patients was waived due to a retrospective nature of our study.
A Bactec FX Instrument (Becton, Dickinson and Company, Sparks, MD, USA) was used for blood cultures. Species identification and antimicrobial susceptibility tests were performed on the VITEK II automated system (bioMérieux, Durham, NC, USA).
The results were analyzed with R version 3.2.3 (December 10, 2015; R Foundation for Statistical Computing, Vienna, Austria). Continuous variables were expressed as the means ± standard deviations and compared with the Student t test or Mann-Whitney U test. Categorical variables were compared with the Pearson chi-square test or Fisher exact test. All tests of significance were two-tailed; p ≤ 0.05 was considered to be significant. Logistic regression analysis was performed to identify risk factors for 7-day and 30-day mortality in community-onset E. coli and K. pneumoniae bacteremia and predictive factors for K. pneumoniae bacteremia. All parameters with a p < 0.2 in the univariate analysis were considered in the multivariable analysis.
A total of 866 patients with COEC and 299 patients with COKP bacteremia were enrolled (Fig. 1). The resistance rates of antimicrobials, which commonly used to treat Gram negative strains, were higher in COEC bacteremia than in COKP bacteremia (Table 1). Isolates from both groups showed high susceptibility to imipenem and amikacin. Increasing trends of 3GC and FQ resistance were observed in both COEC and COKP bacteremia from 2010 to 2016 and the 3GC and FQ resistance rates were higher in COEC bacteremia than in COKP bacteremia throughout the study period (Fig. 2). The FQ resistance rates were higher than the 3GC resistance rates in COEC bacteremia; in contrast, the FQ and 3GC resistance rates were similar in COKP bacteremia.
Comparisons of demographic characteristics, comorbidities and clinical features in patients with the COEC and COKP bacteremia are shown in Table 2. The patients with COEC bacteremia were older than those with COKP bacteremia (mean age, 71.3 ± 14.1 vs. 69.5 ± 14.1, p = 0.049). The COKP bacteremia group had a higher proportion of male patients than the COEC bacteremia group (56.9% vs. 29.7%, p < 0.001). The patients with COKP bacteremia had more severe underlying comorbidities than the patients with COEC bacteremia (CCI, 1.8 ± 2.0 vs. 1.5 ± 1.8, p = 0.035). Ulcer and moderate or severe liver disease were significantly more frequent in patients with K. pneumoniae bacteremia (p < 0.05). Urinary tract infections (UTIs) were more common sources of COEC bacteremia (53.4% vs. 19.7%, p < 0.001), on the other hand, intra-abdominal infections (IAIs) (36.6% vs. 62.2%, p < 0.001) and pneumonia (6.3% vs. 13.0%, p < 0.001) were more common sources of COKP bacteremia. The subgroup analysis showed that the 3GC (18.6% vs. 5.8%, p = 0.006) and FQ (20.3% vs. 5.0%, p < 0.001) resistance rates of COKP bacteremia in UTIs were higher than those in non-UTIs and the 3GC resistance rate (20.9% vs. 14.8%, p = 0.034) of COEC bacteremia in non-IAIs was higher than that in IAIs. The severity at the onset of bacteremia was higher in COKP bacteremia than in COEC bacteremia (PBS, 2.0 ± 2.6 vs. 1.3 ± 1.8, p < 0.001). Although inappropriate antimicrobial therapy was more common in COEC bacteremia than in COKP bacteremia (22.8% vs. 10.0%, p < 0.001) due to higher antimicrobial resistance rates, the outcomes were better in COEC bacteremia than in COKP bacteremia (7-day mortality, 6.9% vs. 12.0%, p = 0.007; 30-day mortality, 8.8% vs. 14.4%, p = 0.008). The Kaplan-Meier survival curve showed consistently higher survival rate within 30 days of bacteremia onset in patients with COEC bacteremia than in patients with COKP bacteremia (p = 0.0304).
Age younger than 70 years, male sex, polymicrobial infections, pneumonia, IAIs, PBS ≥ 2, and Foley catheter insertion were independent predictive factors for COKP bacteremia compared to COEC bacteremia in the multivariate analysis (Table 3). PBS was the only independent risk factor for 7-day mortality in the multivariate analysis (odds ratio, 2.16; 95% confidence interval, 1.92 to 2.46; p < 0.001). CCI, PBS, and intensive care unit (ICU) admission were the independent risk factors for 30-day mortality in the multivariate analysis (Table 4).
When we compared the results of this study with those of our previous study performed from 2003 to 2009 in the same hospital [1], we found that antimicrobial resistance to 3GCs and FQs increased, respectively, from 6.6% to 19.0% and 24.5% to 30.4% in COEC bacteremia and from 4.9% to 8.4% and 4.9% to 8.0% in COKP bacteremia. The resistance rates of E. coli and K. pneumoniae to 3GCs and FQs were much higher in the Korean Anti-microbial Resistance Monitoring System (KARMS) from 2013 to 2015 than in our study because KARMS data includes both nosocomial and community pathogens [7]. In a recent study of community-onset E. coli and Klebsiella species bacteremia from 2012 to 2015, 27.2% of E. coli and 14.9% of Klebsiella species were ESBL producers and the ciprofloxacin resistance rate was 38.7% in E. coli and 21.0% in Klebsiella species [2]; the resistant rates of 3GC and FQs were higher than reported in our studies, especially in the Klebsiella species, because the underlying comorbidities were more severe than those of our studies. Because Daegu Fatima Hospital is a secondary care community-based medical institution, we believe that our data reflects the antimicrobial resistance rates in community-onset infections, and therefore, 3GCs and FQs remain useful empirical options for community- onset infections caused by K. pneumoniae. The antimicrobial susceptibilities in community-acquired K. pneumoniae bacteremia were very high in other Korean and Taiwan studies, which supported our opinion [8,9].
Compared to E. coli bacteremia, K. pneumoniae bacteremia had more underlying comorbidities; higher severity scores at the time of bacteremia onset; other unfavorable clinical characteristics and outcomes, such as ventilator therapy, deterioration of consciousness, ICU admission, and mortality. The multivariate risk factor analysis revealed that only underlying comorbidity (CCI), severity at the time of bacteremia onset (PBS), and ICU admission were significant independent factors for 30-day mortality; appropriateness of antimicrobial therapy and strain type were not independent factors. This is consistent with the results of a propensity score-matched analysis comparing the clinical outcomes of K. pneumoniae and E. coli community-onset monomicrobial bacteremia [3]. That study showed no significant differences in 14- and 28-day crude mortality rates between the two groups after appropriate matching, in which propensity score was calculated by the independent predictors of 28-day crude mortality assessed in a multivariable logistic regression model. In other words, K. pneumoniae causes more severe bacteremia in patients with poorer underlying conditions than E. coli, leading to high mortality. The significant predictive factors for K. pneumoniae bacteremia compared to E. coli bacteremia were age < 70, male sex, polymicrobial infections, pneumonia, IAIs, PBS ≥ 2, and Foley catheter insertion. When treating patients with community-onset Gram-negative bacteremia, it is important for clinicians to predict or know whether the causative micro-organisms are K. pneumoniae or E. coli for antimicrobial selection and prognosis prediction.
One of the reasons for the low mortality rate of E. coli bacteremia is that UTI is the most frequent clinical diagnosis in patients with E. coli bacteremia. A study showed that UTI is a favorable factor for 30-day mortality in E. coli bacteremia [10]. UTI did not show a significant favorable impact on 30-day mortality in our study because of other important factors such as ICU admission, CCI, or PBS. Antimicrobial resistance and inappropriate antimicrobial therapy did not correlate with mortality in our study, probably due to the large proportion of patients with E. coli bacteremia [10]. In some population-based studies, E. coli bacteremia was more frequent in elderly patients who had urological co-morbidities and became more susceptible to UTI [11-13]. In these studies, the male to female ratio was similar; in contrast, in our study and in a Korean study involving patients with acute pyelonephritis, E. coli was more common in women [14].
This study has some limitations. First, it is a retrospective study and was conducted at a single center. Accordingly, our data should be interpreted and applied with caution. Nevertheless, our hospital is the largest secondary care medical institution in the Daegu area and we analyzed more than one thousand cases of E. coli and K. pneumoniae community-onset bacteremia. In our study, the proportion of patients with underlying comorbidities was lower than a community-onset bacteremia study conducted at a tertiary care hospital in Seoul [2]. Therefore, we believe that our data are more representative of community-onset infections than those of studies performed in a tertiary care hospital. Second, we did not distinguish between healthcare-associated and community-acquired cases. In actual clinical situations, it may not be easy to clearly distinguish between the two. Instead, we focused on the comparative analysis of community-onset K. pneumoniae and E. coli bacteremia.
In conclusion, the increasing resistance to 3GCs and FQs in COEC and COKP bacteremia is a serious problem in Korea. Because E. coli and K. pneumoniae are major causative microorganisms in community-onset bacteremia and their resistance to 3GCs and FQs has been increasing, carbapenems and piperacillin/tazobactam are increasingly used [15,16]. Given that E. coli bacteremia is the most common cause of Gram-negative bacteremia and UTI; therefore, clinicians requires attention to use 3GC and FQs as the empirical treatment of patients with probable COEC infections. However, the resistance rates to 3GCs and FQs remain were approximately 5% in COKP bacteremia caused by non-UTI; therefore, clinicians can still use 3GCs or FQs for empirical treatment of patients with probable COKP infections caused by non-UTI, instead of carbapenems or piperacillin/ tazobactam. Appropriate empirical FQs also have the advantage that decreased the duration of fever more than 3GCs in adult community-onset bacteremia [17]. Importantly, COKP bacteremia showed higher mortality, owing to more comorbidities and greater severity, compared to COEC bacteremia. Rapid identification of K. pneumoniae in community-onset bacteremia is helpful for predicting the clinical course and prognosis and selecting appropriate antimicrobials. The results of this comparison of antimicrobial resistance patterns and clinical features in community-onset K. pneumoniae and E. coli bacteremia may be useful for refining diagnostic and treatment strategies.
1. The increasing resistance to 3rd generation cephalosporins (3GCs) and f luoroquinolones (FQs) in community-onset Escherichia coli and Klebsiella pneumoniae bacteremia is a serious problem in Korea.
2. But, 3GCs and FQs are still useful for the empirical treatment of patients with probable community-onset K. pneumonia bacteremia caused by non-urinary tract infections.
3. The results of this study may help to apply the carbapenem sparing strategies to the treatment of community-onset infection
Figure 2.
Trends of third-generation cephalosporin (3GC) and f luoroquinolone (FQ) resistance in community-onset Escherichia coli and Klebsiella pneumoniae bacteremia from 2010 to 2016.
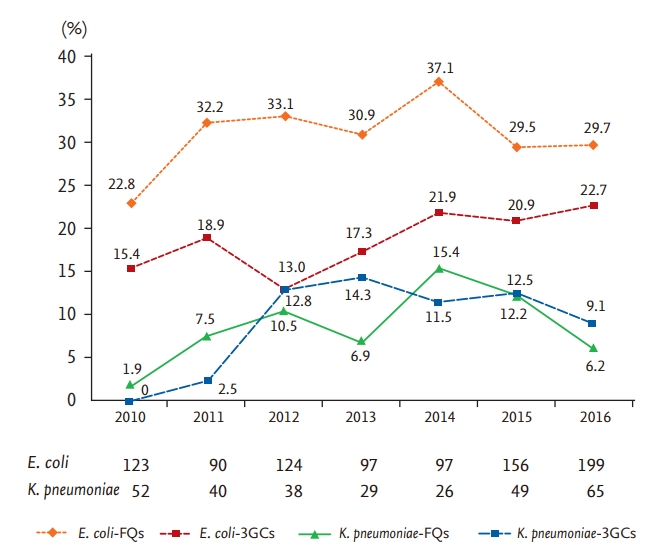
Table 1.
Comparisons of antimicrobial resistance rates in community-onset Escherichia coli and Klebsiella pneumoniae bacteremia
Table 2.
Comparisons of demographic characteristics, comorbidities and clinical features in community-onset Escherichia coli and Klebsiella pneumoniae bacteremia
Table 3.
Predictive factors for Klebsiella pneumoniae in patients with community-onset Escherichia coli or K. pneumoniae bacteremia
Table 4.
Risk factors for 30-day mortality in patients with community-onset Escherichia coli or Klebsiella pneumoniae bacteremia
REFERENCES
1. Lee S, Han SW, Kim KW, Song DY, Kwon KT. Third-generation cephalosporin resistance of community-onset Escherichia coli and Klebsiella pneumoniae bacteremia in a secondary hospital. Korean J Intern Med 2014;29:49–56.




2. Kim M, Song KH, Kim CJ, et al. Clinical prediction score for community-onset bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella species. J Korean Med Sci 2019;34:e116.



3. Kuo TH, Yang CY, Lee CH, Hsieh CC, Ko WC, Lee CC. Propensity score matched analysis comparing the clinical outcome of Klebsiella pneumoniae and Escherichia coli causing community-onset monomicrobial bacteremia. Medicine (Baltimore) 2017;96:e7075.



4. Leistner R, Gurntke S, Sakellariou C, et al. Bloodstream infection due to extended-spectrum beta-lactamase (ESBL)-positive K. pneumoniae and E. coli: an analysis of the disease burden in a large cohort. Infection 2014;42:991–997.



5. Paterson DL, Ko WC, Von Gottberg A, et al. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann Intern Med 2004;140:26–32.


6. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383.

7. Kim D, Ahn JY, Lee CH, et al. Increasing resistance to extended- spectrum cephalosporins, fluoroquinolone, and carbapenem in gram-negative bacilli and the emergence of carbapenem non-susceptibility in Klebsiella pneumoniae: analysis of Korean Antimicrobial Resistance Monitoring System (KARMS) data from 2013 to 2015. Ann Lab Med 2017;37:231–239.



8. Juan CH, Chuang C, Chen CH, Li L, Lin YT. Clinical characteristics, antimicrobial resistance and capsular types of community-acquired, healthcare-associated, and nosocomial Klebsiella pneumoniae bacteremia. Antimicrob Resist Infect Control 2019;8:1.



9. Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis 2012;12:881–887.


10. Cheong HS, Kang CI, Kwon KT, et al. Clinical significance of healthcare-associated infections in community-onset Escherichia coli bacteraemia. J Antimicrob Chemother 2007;60:1355–1360.



11. Bou-Antoun S, Davies J, Guy R, Johnson AP, Sheridan EA, Hope RJ. Descriptive epidemiology of Escherichia coli bacteraemia in England, April 2012 to March 2014. Euro Surveill 2016;21:pii30329.

12. Williamson DA, Lim A, Wiles S, Roberts SA, Freeman JT. Population-based incidence and comparative demographics of community-associated and healthcare-associated Escherichia coli bloodstream infection in Auckland, New Zealand, 2005-2011. BMC Infect Dis 2013;13:385.



13. Laupland KB, Gregson DB, Church DL, Ross T, Pitout JD. Incidence, risk factors and outcomes of Escherichia coli bloodstream infections in a large Canadian region. Clin Microbiol Infect 2008;14:1041–1047.


14. Hyun M, Lee JY, Kim HA, Ryu SY. Comparison of Escherichia coli and Klebsiella pneumoniae acute pyelonephritis in Korean patients. Infect Chemother 2019;51:130–141.



15. Kim B, Myung R, Lee MJ, Kim J, Pai H. Trend of antibiotics usage for acute pyelonephritis in Korea based on National Health Insurance data 2010-2014. BMC Infect Dis 2019;19:554.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



