 |
 |
| Korean J Intern Med > Volume 35(4); 2020 > Article |
|
Abstract
Background/Aims
Methods
Results
Conclusions
Acknowledgments
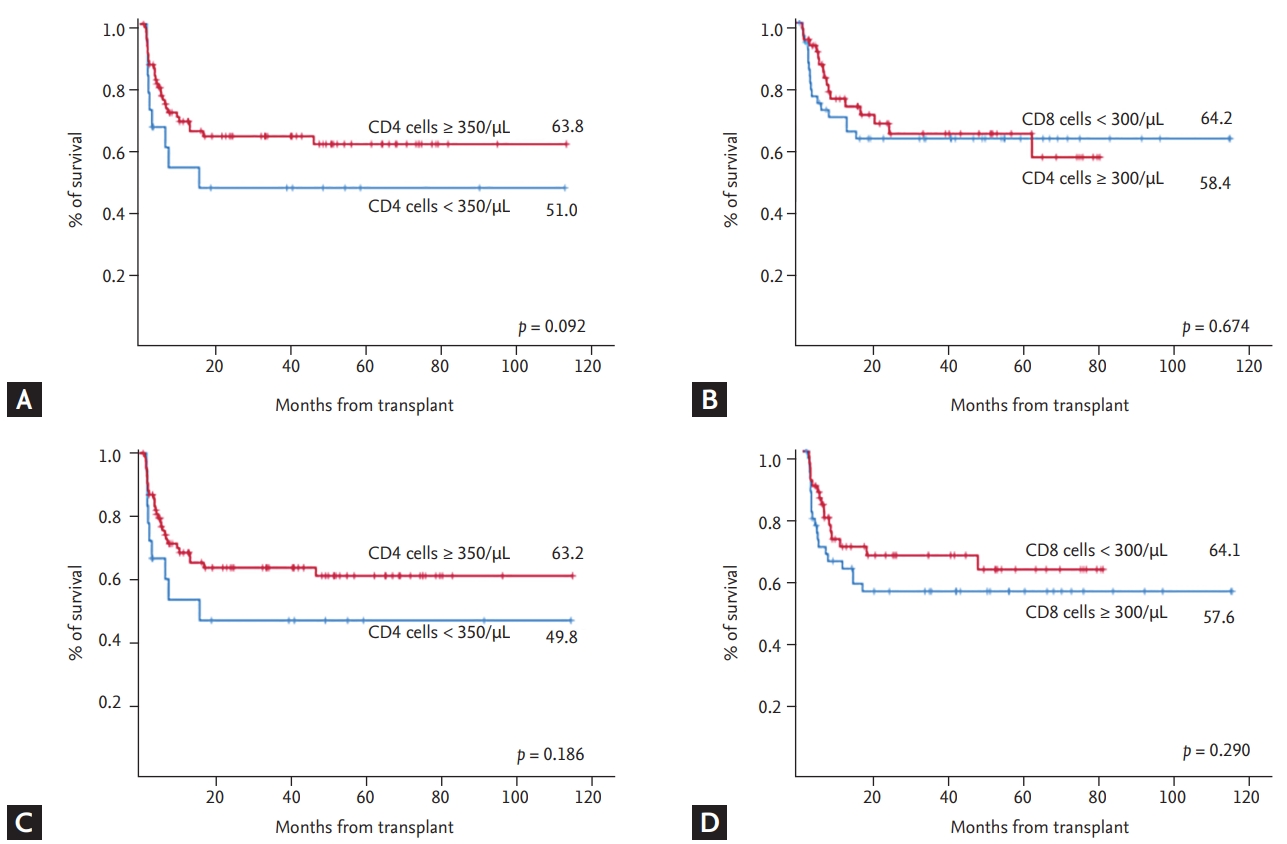
Figure┬Ā1.
Table┬Ā1.
| Variable | Value |
|---|---|
| Age, yr | 43.5 (2ŌĆō71) |
| ŌĆā< 40 | 48 (42.1) |
| ŌĆā40ŌĆō49 | 28 (24.6) |
| ŌĆā50ŌĆō59 | 29 (25.4) |
| ŌĆāŌēź 60 | 9 (7.9) |
| Sex | |
| ŌĆāMale | 62 (54.4) |
| ŌĆāFemale | 52 (45.6) |
| Diagnosis | |
| ŌĆāAML | 59 (51.8) |
| ŌĆāALL | 27 (23.7) |
| ŌĆāMDS | 11 (9.6) |
| ŌĆāLymphoma | 4 (3.5) |
| ŌĆāOthers | 13 (11.4) |
| Cytogenetics | |
| ŌĆāNormal | 64 (56.1) |
| ŌĆāAbnormal | 50 (43.9) |
| Risk stratification by HCT-CI | |
| ŌĆāStandard | 66 (57.9) |
| ŌĆāHigh | 48 (42.1) |
| Disease status at HSCTa | |
| ŌĆāCR | 73 (64.1) |
| ŌĆāNon-CR | 25 (21.9) |
| ŌĆāOthers | 16 (14.0) |
| Conditioning regimen | |
| ŌĆāMyeloablative | 82 (71.9) |
| ŌĆāReduced intensity/non-myeloablative | 32 (28.1) |
| Type of allogeneic HSCT | |
| ŌĆāMatched related donor | 30 (26.3) |
| ŌĆāMatched unrelated donor | 68 (59.7) |
| ŌĆāHaploidentical donor | 16 (14.0) |
| T-cell depletion | |
| ŌĆāYes | 78 (68.4) |
| ŌĆāNo | 36 (31.6) |
| Prophylaxis for acute GVHD | |
| ŌĆāCyclosporin-A based regimen | 22 (19.3) |
| ŌĆāTacrolimus based regimen | 92 (80.7) |
Values are presented as median (range) or number (%).
AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; HCT-CI, hematopoietic cell transplantation-comorbidity index; HSCT, hematopoietic stem cell transplantation; CR, complete remission; GVHD, graft-versus-host disease.
Table┬Ā2.
Table┬Ā3.
| Variable |
Overall survival |
||
|---|---|---|---|
| HR | 95% CI | p value | |
| Time of HSCT | |||
| ŌĆā2001ŌĆō2008 | 0.740 | ||
| ŌĆā2009ŌĆō2014 | 0.83 | 0.43ŌĆō1.62 | |
| ŌĆāŌēź 2015 | 1.10 | 0.49ŌĆō2.46 | |
| Sex | |||
| ŌĆāMale | 0.249 | ||
| ŌĆāFemale | 0.70 | 0.38ŌĆō1.28 | |
| Age, yr | |||
| ŌĆā< 40 | 0.486 | ||
| ŌĆā40ŌĆō49 | 1.29 | 0.64ŌĆō2.61 | |
| ŌĆā50ŌĆō59 | 0.91 | 0.43ŌĆō1.89 | |
| ŌĆāŌēź 60 | 0.39 | 0.04ŌĆō2.21 | |
| Disease type | |||
| ŌĆāAML | 0.255 | ||
| ŌĆāALL | 1.47 | 0.75ŌĆō2.88 | |
| ŌĆāMDS | 0.00 | 0.00ŌĆō1.11 | |
| ŌĆāLymphoma | 3.90 | 1.16ŌĆō13.14 | |
| ŌĆāOthers | 1.40 | 0.56ŌĆō3.45 | |
| Disease status at HSCTa | |||
| ŌĆāCR | 0.034 | ||
| ŌĆāNon-CR | 2.54 | 1.07ŌĆō5.99 | |
| Risk stratification by HCT-CI | |||
| ŌĆāStandard | 0.000 | ||
| ŌĆāHigh | 2.94 | 1.62ŌĆō5.36 | |
| Donor type | |||
| ŌĆāMatched related donor | 0.656 | ||
| ŌĆāMatched unrelated donor | 0.73 | 0.38ŌĆō1.44 | |
| ŌĆāHaplo-identical donor | 0.88 | 0.33ŌĆō2.30 | |
| Conditioning | |||
| ŌĆāMyeloablative | 0.662 | ||
| ŌĆāRIC/NST | 1.16 | 0.60ŌĆō2.25 | |
| T-cell depletion | |||
| ŌĆāNo | 0.136 | ||
| ŌĆāYes | 1.69 | 0.35ŌĆō1.32 | |
| Prophylaxis for acute GVHD | |||
| ŌĆāWith cyclosporin-A | 0.259 | ||
| ŌĆāWith tacrolimus | 0.68 | 0.35ŌĆō1.32 | |
| Grade IIŌĆōIV acute GVHD | |||
| ŌĆāNo | 0.068 | ||
| ŌĆāYes | 1.74 | 0.96ŌĆō3.14 | |
| Chronic GVHD | |||
| ŌĆāNo | 0.006 | ||
| ŌĆāYes | 0.30 | 0.13ŌĆō0.70 | |
A p values were calculated by Cox-proportional hazards regression analysis.
HR, hazard ratio; CI, conf idence interval; HSCT, hematopoietic stem cell transplantation; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; CR, complete remission; HCT-CI, hematopoietic cell transplantation-comorbidity index; RIC/NST, reduced intensity conditioning/non-myeloablative stem transplantation; GVHD, graft-versus-host disease.
Table┬Ā4.
Table┬Ā5.
| Variable | Total (n = 104) | Normal (n = 39) | Abnormal (n = 65) | p value |
|---|---|---|---|---|
| Age, yr | 0.633 | |||
| ŌĆā< 40 | 44 (42.3) | 19 (48.7) | 25 (38.5) | |
| ŌĆā40ŌĆō49 | 25 (24.0) | 10 (25.6) | 15 (23.1) | |
| ŌĆā50ŌĆō59 | 27 (26.0) | 8 (20.5) | 19 (29.2) | |
| ŌĆāŌēź 60 | 8 (7.7) | 2 (5.1) | 6 (9.2) | |
| Sex | 1.000 | |||
| ŌĆāMale | 54 (51.9) | 20 (51.3) | 34 (52.3) | |
| ŌĆāFemale | 50 (48.1) | 19 (48.7) | 31 (47.7) | |
| Diagnosis | 0.900 | |||
| ŌĆāAML | 53 (51.0) | 19 (48.7) | 34 (52.3) | |
| ŌĆāALL | 23 (22.1) | 10 (25.6) | 13 (20.0) | |
| ŌĆāMDS | 11 (10.6) | 3 (7.7) | 8 (12.3) | |
| ŌĆāLymphoma | 3 (2.9) | 1 (2.6) | 2 (3.1) | |
| ŌĆāOthers | 14 (13.5) | 6 (15.4) | 8 (12.3) | |
| Disease status at transplanta | 0.381 | |||
| ŌĆāCR | 66 (63.5) | 23 (59.0) | 43 (66.2) | |
| ŌĆāNon-CR | 22 (21.2) | 11 (28.2) | 11 (16.9) | |
| ŌĆāOthers | 16 (15.4) | 5 (12.8) | 11 (16.9) | |
| HCT-CI risk | 0.165 | |||
| ŌĆāStandard | 61 (58.7) | 19 (48.7) | 42 (64.6) | |
| ŌĆāHigh | 43 (41.3) | 20 (51.3) | 23 (35.4) | |
| Conditioning intensity | 0.434 | |||
| ŌĆāMA | 74 (71.2) | 30 (76.9) | 44 (67.7) | |
| ŌĆāRIC/NST | 30 (28.8) | 9 (23.1) | 21 (32.3) | |
| Prevention of GVHD | 0.038 | |||
| ŌĆāWith cyclosporin-A | 20 (19.2) | 12 (30.8) | 8 (12.3) | |
| ŌĆāWith tacrolimus | 84 (80.8) | 27 (69.2) | 57 (87.7) | |
| Donor type | 0.31 | |||
| ŌĆāMatched related donor | 26 (25.0) | 11 (28.2) | 15 (23.1) | |
| ŌĆāMatched unrelated donor | 63 (60.6) | 25 (64.1) | 38 (58.5) | |
| ŌĆāHaplo-identical donor | 15 (14.4) | 3 (7.7) | 12 (18.5) | |
| T-cell depletion | <0.001 | |||
| ŌĆāNo | 29 (28.9) | 20 (69.0) | 9 (31.0) | |
| ŌĆāYes | 75 (72.1) | 19 (48.7) | 56 (86.2) | |
| ŌĆāInfused CD34+ cells (> 6 ├Ś 106/kg) | 58 (55.8) | 19 (48.7) | 39 (60.0) | 0.359 |
Values are presented as number (%). A p values were calculated by chi-square test or FisherŌĆÖs exact test.
AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; CR, complete remission; HCT-CI, hematopoietic cell transplantation-comorbidity index; MA, myeloablative; RIC/NST, reduced intensity conditioning/non-myeloablative stem cell transplant; GVHD, graft-versus-host disease.
Table┬Ā6.
Table┬Ā7.
Table┬Ā8.
Table┬Ā9.
| Variable | Total (n = 108) | Normal (n = 97) | Abnormal (n = 11) | p value |
|---|---|---|---|---|
| Age, yr | 0.109 | |||
| ŌĆā< 40 | 45 (41.7) | 38 (39.2) | 7 (63.6) | |
| ŌĆā40ŌĆō49 | 26 (24.1) | 23 (23.7) | 3 (27.3) | |
| ŌĆā50ŌĆō59 | 29 (26.9) | 29 (29.9) | 0 | |
| ŌĆāŌēź 60 | 8 (7.4) | 7 (7.2) | 1 (9.1) | |
| Sex | 0.846 | |||
| ŌĆāMale | 57 (52.8) | 52 (53.6) | 5 (45.5) | |
| ŌĆāFemale | 51 (47.2) | 45 (46.4) | 6 (54.5) | |
| Diagnosis | 0.727 | |||
| ŌĆāAML | 54 (50.0) | 50 (51.5) | 4 (36.4) | |
| ŌĆāALL | 26 (24.1) | 22 (22.7) | 4 (36.4) | |
| ŌĆāMDS | 11 (10.2) | 10 (10.3) | 1 (9.1) | |
| ŌĆāLymphoma | 3 (2.8) | 3 (3.1) | 0 | |
| ŌĆāOthers | 14 (13.0) | 12 (12.4) | 2 (18.2) | |
| Disease status at transplantationa | 0.294 | |||
| ŌĆāCR | 69 (63.9) | 64 (66.0) | 5 (45.5) | |
| ŌĆāNon-CR | 23 (21.3) | 19 (19.6) | 4 (36.4) | |
| ŌĆāOthers | 16 (14.8) | 14 (14.4) | 2 (18.2) | |
| HCT-CI risk | 0.520 | |||
| ŌĆāStandard | 63 (58.3) | 58 (59.8) | 5 (45.5) | |
| ŌĆāHigh | 45 (41.7) | 39 (40.2) | 6 (54.5) | |
| Conditioning intensity | 0.434 | |||
| ŌĆāMA | 77 (71.3) | 70 (72.2) | 7 (63.6) | |
| ŌĆāRIC/NST | 31 (28.7) | 27 (27.8) | 4 (36.4) | |
| Donor type | 0.399 | |||
| ŌĆāMatched related donor | 30 (27.8) | 28 (28.9) | 2 (18.2) | |
| ŌĆāMatched unrelated donor | 63 (58.3) | 57 (58.8) | 6 (54.5) | |
| ŌĆāHaplo-identical donor | 15 (13.9) | 12 (12.4) | 3 (27.3) | |
| T-cell depletion | 1.000 | |||
| ŌĆāNo | 32 (28.9) | 29 (28.4) | 3 (27.3) | |
| ŌĆāYes | 76 (71.7) | 68 (71.6) | 8 (72.7) | |
| Infused CD34+ cells (> 6 ├Ś 106/kg) | 58 (53.7) | 59 (60.8) | 3 (27.2) | 0.054 |
| Infused CD34+ cells (Ōēż 6 ├Ś 106/kg) | 50 (46.3) | 38 (39.2) | 8 (72.7) |
Values are presented as number (%). A p values were calculated by chi-square test or FisherŌĆÖs exact test.
NK, natural killer; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; CR, complete remission; HCT-CI, hematopoietic cell transplantation-comorbidity index; MA, myeloablative; RIC/NST, reduced intensity conditioning/non-myeloablative stem cell transplant.
REFERENCES
-
METRICS

- Related articles
-
Immunologic and non-immunologic complications of a third kidney transplantation2015 September;30(5)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


