INTRODUCTION
Interstitial lung diseases are diffuse parenchymal lung disorders associated with lung inflammation and excessive deposition of extracellular matrix, eventually leading to the destruction of lung structure and loss of lung function [1]. Among these diseases, idiopathic pulmonary fibrosis (IPF) is the most lethal and presents with high heterogeneity in its clinical manifestation, with a reported median survival time of approximately 2.8 years [2].
MicroRNAs (miRNAs) are a class of small noncoding RNAs, approximately 18 to 22 nucleotides in length [3], that regulate the expression of target genes involved in various physiological processes.
It has been demonstrated that miRNAs play important roles in the pathogenesis of pulmonary diseases [4]. The therapeutic effect of an antagomir targeting miR-30a, at reducing pulmonary fibrosis, has been validated in vivo [5], indicating that miRNAs may become the next class of therapeutics. However, studies exploring the therapeutic efficacy of single-stranded miRNA inhibitors, known as antagomirs, in IPF are lacking. In this study, microarray analysis was used to investigate the changes in miRNA expression in lung tissue in a mouse model of IPF induced by bleomycin (BLM). miR-155 was identified as an miRNA up-regulated during the development of pulmonary fibrosis. Therefore, we aimed to explore whether in vivo silencing of miR-155, using a synthetic miRNA inhibitor (antagomiR-155), could impede the development of pulmonary fibrosis by targeting a certain signaling pathway.
METHODS
Mice
Six-week-old male C57BL/6J (B6) mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and were housed in a pathogen-free environment in the animal facilities of Renji Hospital, affiliated with the Shanghai Jiaotong University School of Medicine (Shanghai, China). All mouse procedures were approved by the Animal Care and Use Committee of Renji Hospital, Shanghai Jiaotong University, School of Medicine (RJ-2018-03-05).
Mouse model of bleomycin-induced pulmonary fibrosis
Six mice were randomly divided into the following two groups: (1) a BLM group, treated with intratracheal injection of 5 mg/kg BLM (15 mg BLM dissolved in 15 mL of phosphate-buffered saline [PBS]; Hisun-Pfizer Pharmaceuticals, Shanghai, China) on day 1 [6] and (2) a control group, treated with intratracheal injection of the same volume of PBS on day 1. Mice were sacrificed on day 18 after being anesthetized with an intraperitoneal injection of 3% chloral hydrate (0.01 mL/g). The left lung lobe was embedded in paraffin for sectioning, while the right lung lobe was microdissected for total RNA extraction and miRNA microarray analysis.
Histopathological analysis
Lung tissue samples were fixed with a 4% paraformaldehyde neutral buffer solution for 24 hours, dehydrated in a graded ethanol series, embedded in paraffin, and sectioned at a thickness of 2 to 3 ╬╝m. Paraffin sections were processed with hematoxylin and eosin (H&E) and MassonŌĆÖs trichrome staining. Lung fibrosis was semi-quantified by systematically scanning stained sections under a microscope using 4├Ś, and 10├Ś objectives. Each successive field was individually assessed for the severity of interstitial fibrosis and was allotted a score between 0 (normal lung) and 8 (total coverage of the field with fibrous), using a predetermined Ashcroft scale of severity [7]. After examining the entire section, the mean score of all examined fields was taken as the fibrosis score for the section. X.S. and D.T. assessed the sections and allotted scores independently. Their results were then averaged to determine the final fibrosis score. Both X.S. and D.T. were blinded to the experimental groups.
Total RNA extraction and miRNA microarray analysis
Total RNA was isolated from mouse tissues using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturerŌĆÖs instructions. The quantity and integrity of the extracted RNA were assessed using a Qubit 2.0 instrument (Life Technologies, Carlsbad, CA, USA) and an Agilent 2200 TapeStation (Agilent Technologies, USA), respectively. One microgram of total RNA from each sample was used to prepare RNA libraries using an NEBNext Multiplex Small RNA Library Prep Set for Illumina (New England Biolabs, Ipswich, MA, USA). RNA samples extracted from 3 mice in the same group were pooled and then assayed on a single microarray. The libraries were sequenced on a HiSeq 2500 instrument (Illumina, San Diego, CA, USA) with single-end 50-bp reads, at Ribobio Co. Ltd. (Guangzhou, China).
Candidate miRNA selection
BLM induced significant, extensive alveolar wall thickening; massive infiltration of inflammatory cells into the interstitium; and collagen deposition at day 18 (Supplementary Fig. 1B, 1D, 1F, and 1H), compared with control group (Supplementary Fig. 1A, 1C, 1E, 1G). The semi-quantitative fibrosis scores for the two groups are presented in Supplementary Fig. 1I.
Using a single miRNA microarray, 621 miRNAs were found to be expressed above background and 64 miRNAs showed |log2 (fold change)| Ōēź 1 and p < 0.05, after BLM administration, when compared with the control group. Among these miRNAs, 47 showed elevated expression in the BLM group compared with the control group (Supplementary Table 1, Supplementary Fig. 2). The results of hierarchical clustering analysis are shown in Supplementary Fig. 2. miR-155-3p and miR-155-5p showed high levels of expression with |log2 (fold change)| = 6.8786 and 1.6346, respectively (p < 0.05). Based on previous studies, miR-155 was selected for further investigation.
AntagomiR-155 transfection.
AntagomiR-155 (5ŌĆÖ-ACCCCUAUCACAAUUAGCAUUAA-3ŌĆÖ) was purchased from Ribobio. The Entranster in vivo transfection reagent was obtained from Engreen Biosystem Co. Ltd. (Auckland, New Zealand). Following the manufacturerŌĆÖs instructions, we first prepared reagent A by dissolving 85 nmol of antagomiR-155 in 570 ╬╝L of autoclaved double-distilled H2O and then adding 570 ╬╝L of sterile 10% glucose and mixing well. Next, we prepared reagent B by adding 570 ╬╝L of sterile 10% glucose to 570 ╬╝L of Entranster in vivo transfection reagent and mixing well. Finally, reagents A and B were mixed (1:1) to yield the working solution. Each 330 ╬╝L aliquot of working solution contained 12 nmol of antagomiR-155.
Study design
Nineteen 6-week-old male mice were randomly divided into the following three groups: 1) a BLM+antagomiR-155 group (n = 7), treated with intratracheal injection of 5 mg/kg BLM on day 1, plus intravenous injection of antagomiR-155; 2) a BLM group (n = 7), treated with intratracheal injection of BLM plus intravenous injection of PBS; and 3) a control group (n = 5), treated with intratracheal plus intravenous injection of PBS. Mice in the BLM + antagomiR-155 group received tail vein injections of 330 ╬╝L of an antagomiR-155 working solution on day 2, 3, 4, 14, 15, and 16 after BLM injection (Fig. 1). After sacrifice on day 18, serum samples were collected and stored at ŌĆō20┬░C for subsequent cytokine measurement. The left lung lobe was embedded in paraffin for further histological analysis and the right lung lobe was microdissected, placed into liquid nitrogen, and stored at ŌĆō80┬░C for subsequent polymerase chain reaction (PCR), hydroxyproline (Hyp) measurement, and Western blotting.
Quantitative polymerase chain reaction
miRNA-155 was quantified in lung tissue by TaqMan quantitative polymerase chain reaction (qPCR), according to the manufacturerŌĆÖs protocol (Life Technologies). U6, a reference small nucleolar RNA, was used as an internal control. The primer sequences used were as follows: 5ŌĆ▓-TTAATGCTAATTGTGATAGGGGT-3ŌĆ▓ (miRNA-155); 5ŌĆ▓-CGCAAATTCGTGAAGCGTTC-3ŌĆ▓ (U6). The qPCR reactions were performed in a LightCycler 480 Real-Time PCR System (Roche Applied Science, Penzberg, Germany).
Hydroxyproline assessment
Lung tissue samples (30 to 100 mg wet weight) were lysed in radioimmunoprecipitation assay lysis buffer (JRDUN Bio Co., Ltd., Shanghai, China) at 4┬░C for 30 minutes. The resulting lysates were centrifuged at 3,500 ├Śg at 4┬░C for 10 minutes and total protein levels in the supernatants were quantified using a bicinchoninic acid (BCA) Protein assay kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Hyp content in lung tissue extracts was evaluated using a Hyp assay kit (Sengbeijia Bioengineering Institute, Shanghai, China), according to the manufacturerŌĆÖs instructions and results were read on a microplate reader (BioTek Instruments Inc., Winooski, VT, USA) at a wavelength of 450 nm.
Measurement of cytokine levels in serum by ELISA
The serum concentrations of transforming growth factor-╬▓ (TGF-╬▓), interferon-╬│ (IFN-╬│), and interleukin 4 (IL-4) were measured using enzyme-linked immunosorbent assay (ELISA) kits (Sigma, St Louis, MO, USA), according to the manufacturerŌĆÖs instructions.
Western blotting
The potential targets of miRNA-155 were predicted using miRand, miRWalk 2.0, TargetScan, and miRbase [8,9]. Based on these analyses, SMAD2, TGF-╬▓-activated kinase 1/mitogen-activated protein kinase kinase kinase 7 (MAP3K7)-binding protein 2 (TAB2), and suppressor of cytokine signaling 1 (SOCS1) were selected to investigate the molecular signaling pathways underlying pulmonary fibrosis.
Frozen lung tissues were homogenized in RIPA buffer (pH 7.6) and total protein concentrations were quantified by BCA assay. Equal amounts of protein (20 to 40 ┬Ąg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretically transferred to a polyvinylidene difluoride membrane (Millipore, Burlington, MA, USA). The membranes were blocked and then incubated with primary antibodies against TAB2 (1:1,000; Proteintech, Rosemont, IL, USA), SOCS1 (1:1,000; Cell Signaling Technology, Danvers, MA, USA), SMAD2 (1:1,000; Abways, Shanghai, China). The relative levels of these proteins were determined using image analysis software and were normalized to the levels of ╬▓-actin (1:3,000; Abways). The experiments were repeated three times.
Statistical analysis
Data analysis was performed using Prism software, version 6.0c (GraphPad, San Diego, CA, USA). Continuous variables are represented as the mean ┬▒ SE or median. A StudentŌĆÖs t test or one-way analysis of variance was used to determine the differences between mean values for normally distributed variables. The nonparametric Mann-Whitney U test or the Kruskal-Wallis test was used to determine the differences between non-normally distributed variables. A p values less than 0.05 (2-tailed) were considered statistically significant. For microarray data, edgeR was used to compare miRNA levels between the BLM group and the control group. |log2 (fold change)| Ōēź 1 and p < 0.05 were considered statistically significant.
RESULTS
Mouse survival and body weight
Two mice from the BLM group and two from the BLM+ antagomiR-155 group died at day 2 (before antagomiR-155 injection) and were excluded from the study. At the end of the study, the body weight of the BLM group decreased from 20.6 ┬▒ 0.4 g to 19.0 ┬▒ 1.3 g, which was significantly lower than the body weight of the BLM + antagomiR-155 group (20.5 ┬▒ 0.3 g, p = 0.01) and the control group (23.7 ┬▒ 0.3 g, p < 0.001) (Fig. 2).
Attenuated bleomycin-induced pulmonary fibrosis following in vivo silencing of miR-155
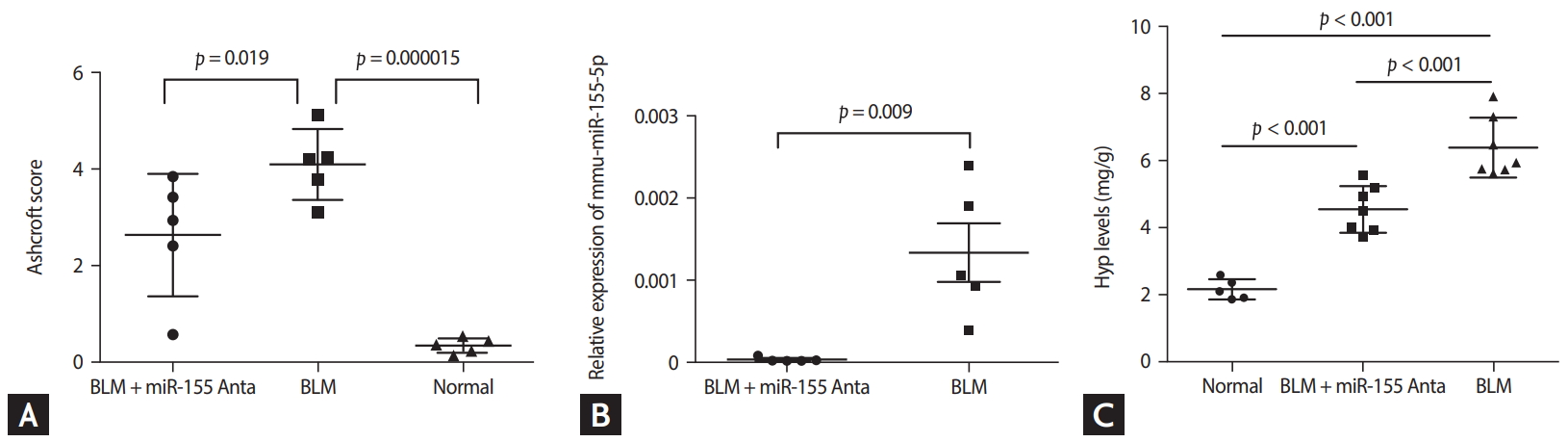
Histopathological analysis showed that compared with control group (Fig. 3A, 3D, 3G, 3J), all BLM-treated mice developed pulmonary fibrosis (Fig. 3B, 3E, 3H, and 3K). However, treatment with antagomiR-155 significantly inhibited the alveolar wall thickening, infiltration of inflammatory cells into the interstitium, and reduction in collagen deposition induced by BLM (Fig. 3C, 3F, 3I, and 3L). A comparison of Ashcroft scores demonstrated that the degree of pulmonary fibrosis in the BLM group was markedly greater than in the control group, but was impeded by antagomiR-155 treatment (Fig. 4A), indicating that pathomorphological sequelae of BLM-induced pulmonary fibrosis could be attenuated by in vivo silencing of miR-155 with antagomiR-155.
Downregulated miRNA-155 expression in target tissues
As shown in Fig. 4B, antagomiR-155 significantly downregulated the expression levels of miR-155 in lung tissues at day 18.
Hydroxyproline levels
Hyp content in lung tissues was measured as a representative marker of collagen deposition. As demonstrated in Fig. 4C, Hyp levels were significantly elevated in the BLM group (p < 0.001) and this effect was inhibited by antagomiRNA-155 (p < 0.001).
Changes in serum cytokine levels
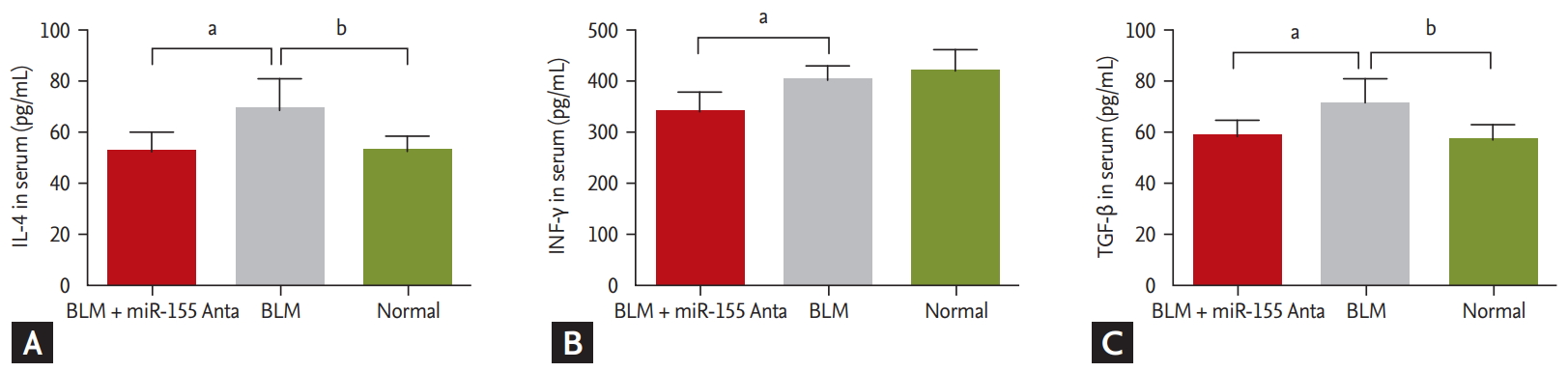
Serum levels of the profibrogenic cytokines, IL-4 and TGF-╬▓, were significantly increased in BLM-treated mice compared with control mice (68.99 ┬▒ 12.03 pg/mL vs. 52.53 ┬▒ 5.94 pg/mL and 70.21 ┬▒ 8.78 pg/mL vs. 56.97 ┬▒ 5.13 pg/mL, respectively; p < 0.05 for both). However, after silencing miRNA-155 in vivo, IL-4, IFN-╬│, and TGF-╬▓ levels were all decreased compared with their levels in the BLM group (52.56 ┬▒ 7.57 pg/mL vs. 68.99 ┬▒ 12.03 pg/mL, 355.89 ┬▒ 43.63 pg/mL vs. 406.24┬▒25.57 pg/mL, and 57.15 ┬▒ 6.82 pg/mL vs. 70.21 ┬▒ 8.78 pg/mL, respectively; p < 0.05 for all) (Fig. 5).
miRNA-155-associated molecular signaling pathways underly bleomycin-induced pulmonary fibrosis in mice
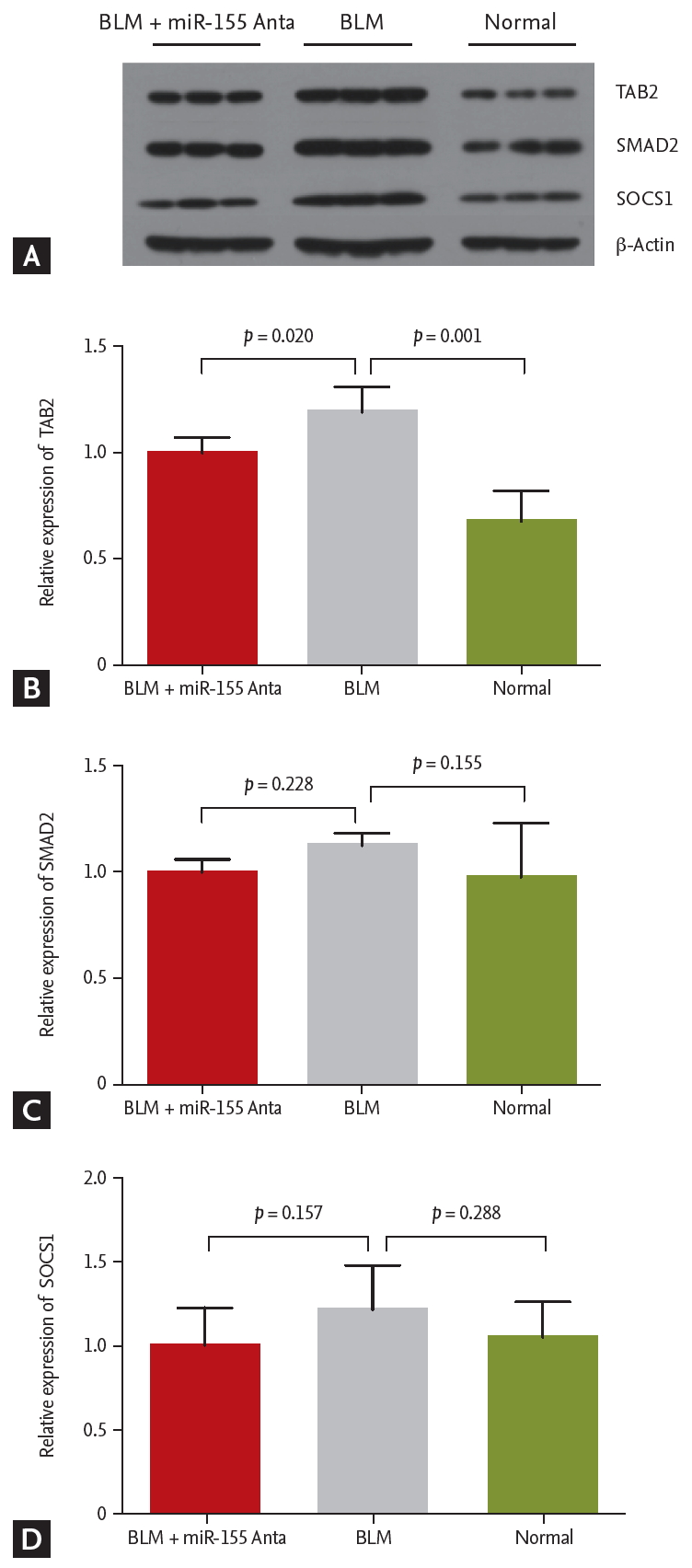
We assessed the expression levels of proteins involved in various signaling pathways (SMAD2, TAB2, SOCS1). Among those tested, only TAB2 was found to be activated by BLM and inhibited by miR-155 silencing in vivo (Fig. 6).
DISCUSSION
Pulmonary fibrosis is characterized by the presence of fibroblastic foci and myofibroblasts that produce extracellular matrix components, such as collagen type I and III and fibronectin [10]. Intratracheal administration of BLM in B6 mice is the most commonly used model of pulmonary fibrosis and it may also mimic IPF. It has been shown that pulmonary fibrosis develops within 4 weeks after BLM administration, with collagen deposition increasing rapidly after day 10 and peaking on day 21 [6]. However, the development of fibrosis is partially reversible, independent of any intervention [11]. Therefore, in the present study, we assessed the degree of pulmonary fibrosis on day 18. Our analysis showed that lung tissue structural damage, inflammatory cell infiltration, and collagen deposition in the BLM-treated group were similar to what has been reported in a previous study [6].
Studies on the pathogenesis of pulmonary fibrosis have focused on mechanisms regulating the proliferation and activation of myofibroblasts. It is well accepted that microRNAs are important players in the development of fibrosis in multiple organs, including the heart, liver, kidney, and lung [12,13]. Using microarray analysis technology, approximately 10% of assayed microRNAs have been reported to be significantly altered in IPF lungs [4,14]. We demonstrated that miR-155 expression was significantly increased during the development of pulmonary fibrosis. Previously, we found that the progression of acute lung inflammation in lupus is reduced in miR-155-/- mice and after in vivo silencing of miR-155 [15]. Other studies showed that miR-155 is closely associated with epithelial-mesenchymal interactions [16]. In lung biopsies from patients with IPF, miR-155 is significantly upregulated, in both rapidly progressing and slowly progressing IPF [17]. High expression levels of miR-155 are correlated with the development and the degree of pulmonary fibrosis [12]. These previous results prompted us to select miRNA-155 as a novel target.
Recent clinical efficacy data have underscored the relevance of miRNAs to disease states and the potential for miRNAs to become the next class of therapeutics [18]. Since a single miRNA can regulate numerous target mRNAs within biological pathways, modulation of an miRNA, in principle, allows for the influencing of an entire gene network and the modifying of complex disease phenotypes [19]. Antagomirs are synthetic miRNA inhibitors that are considered to be powerful tools for manipulating miRNA levels in vivo. Studies have shown that the injection of antagomirs, in general, is a feasible approach to efficiently down-regulate specific miRNAs in target tissues in a variety of diseases [20,21]. In the present study, repeated intravenous injections of antagomiR-155 significantly down-regulated the expression levels of miR-155 in lungs, as assessed by qPCR. This attenuated BLM-induced inflammation and pulmonary fibrosis. To the best of our knowledge, our study is the first to report the effect of antagomir-155 on BLM-induced pulmonary fibrosis in vivo. Therefore, we propose that antagomir injection is a feasible approach to inhibit the expression of distinct miRNAs in target tissues and antagomir-155 may provide a novel therapeutic strategy for pulmonary fibrosis, by inhibiting miRNA-155 in pulmonary tissues.
In view of the molecular pathogenesis of pulmonary fibrosis, we further investigated changes in serum cytokine levels during antagomiR-155 treatment of mice with pulmonary fibrosis. The most important function of CD4+ T cells is to produce a large quantity of various cytokines, which may contribute to either the inhibition or promotion of fibroblast proliferation, protein synthesis, and collagen production [22]. In the present study, we studied the following 3 cytokines: IL-4, an important initiator in the Th-2-dominated immune response, inducing TGF-╬▓ production; IFN-╬│, a positive feedback that reinforces the Th1-dominated immune response, inhibiting the production of anti-inflammatory cytokines and promoting the secretion of proinflammatory cytokines; and TGF-╬▓, an important pro-fibrogenic cytokine. All of these cytokines are essential players in the proliferation and differentiation of fibroblasts, the expression of collagen and fibronectin, tenascin synthesis, and epithelial-mesenchymal transition [23-25]. We demonstrated that, after BLM exposure, IL-4 and TGF-╬▓ levels increased significantly, while IFN-╬│ levels remained stable. This may be attributed to the small study sample size. However, in the context of clinical IPF, a number of studies of biopsy material have shown that the overall cytokine pattern is more Th2-type than Th1-type [26,27]. Wallace and Howie [26] immunohistochemically examined diffusely infiltrating cells within the interstitium of patients with IPF and showed that most of the diffusely infiltrating mononuclear cells were positive for IL-4 and IL-5 and a small minority of the cells were positive for IFN-╬│. This may partly explain the different cytokine patterns induced by BLM. Recently, emerging evidence has shown that miRNA-155, a typical multifunctional miRNA, is involved in Th cell differentiation, Th1 and Th2 response regulation, and cytokine production [28,29]. In our study, we showed that IL-4, IFN-╬│, and TGF-╬▓ were all reduced by antagomir-155 treatment, which agrees with previous findings that both the Th1-dominated and Th-2 dominated immune responses are suppressed after miR-155 inhibition [30,31]. This may have contributed to the inhibitory effect of antagomir-155 on pulmonary fibrosis.
TGF-╬▓, which can be induced by IL-4, is a pivotal pro-fibrogenic cytokine. Stimulation of the expression of a number of proinflammatory and fibrogenic cytokines, such as TNF-╬▒ and IL-6, by TGF-╬▓-induced signaling, is firmly established as a central mediator of pulmonary fibrosis [32,33]. A growing body of evidence shows that TGF-╬▓1 activates various SMAD-independent signaling pathways, with or without direct cross-talk with SMAD, and that TGF-╬▓-activated kinase 1 (TAK1)/MAP3K7 is a major upstream signaling molecule in TGF-╬▓-induced type I collagen and fibronectin expression [34,35]. The interaction with TAK1/MAP3K7-binding proteins (TABs) is an essential step for TAK1 activation and is necessary for TGF-╬▓ signal transduction, involved in the activation of inflammatory pathways [36,37]. In the present study, BLM led to a significant increase in the levels of TGF-╬▓ and TAB2, which was in accordance with the aforementioned studies [32,33], thus revealing the role of the TGF-╬▓/TAK1-TAB2 signaling pathway in BLM-induced lung fibrosis. This effect could be significantly suppressed by antagomiR-155 treatment. As previously reported, miR-155 has a direct influence on TGF-╬▓ levels and the knockdown of miR-155 inhibits TGF-╬▓1 signaling activation, which may have contributed to the inhibitory effect of antagomiR-155 on pulmonary fibrosis [30,38].
In conclusion, our findings indicated that miR-155 enhanced the inflammatory responses during the development of BLM-induced fibrosis, by regulating multiple inflammatory factors and signaling pathways. We propose that miR-155 antagonists have a potential therapeutic role in pulmonary fibrosis.
KEY MESSAGE
1. Interleukin 4 (IL-4) and transforming growth factor-╬▓ (TGF-╬▓) expression increased and TGF-╬▓-activated kinase 1/MAP3K7-binding protein 2 (TAB2), of the mitogen-activated protein kinase (MAPK) signaling pathway, were activated in a pulmonary fibrosis model.
2. Intravenous injection of antagomiR-155 reduced miRNA-155 levels in lung tissue.
3. AntagomiR-155 alleviated the pathological changes of pulmonary fibrosis.
4. AntagomiR-155 inhibited IL-4, TGF-╬▓, and interferon-╬│ and TAB2 expression.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print





