 |
 |
| Korean J Intern Med > Volume 36(2); 2021 > Article |
|
Abstract
Background/Aims
Methods
Results
Supplementary Material
Supplementary Figure 1.
Figure 1.

Figure 2.
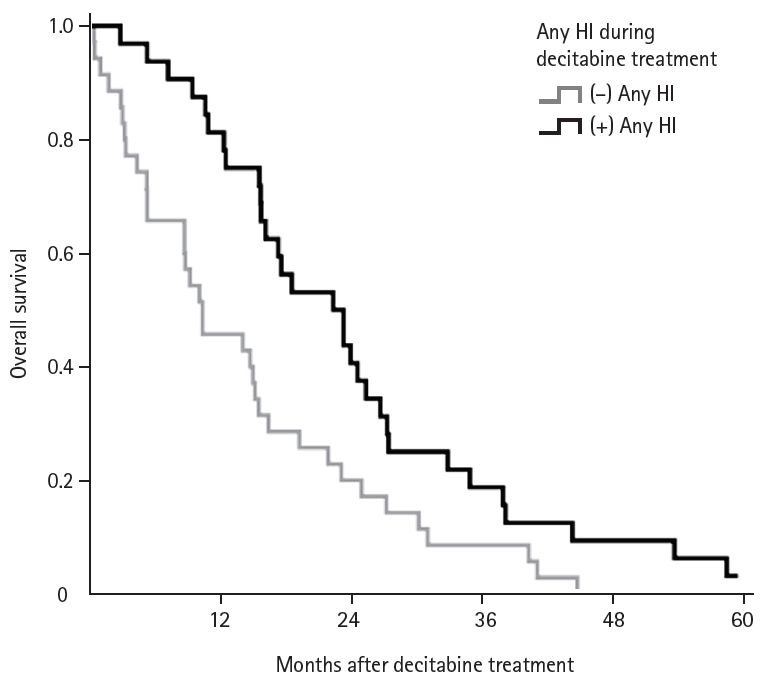
Figure 3.
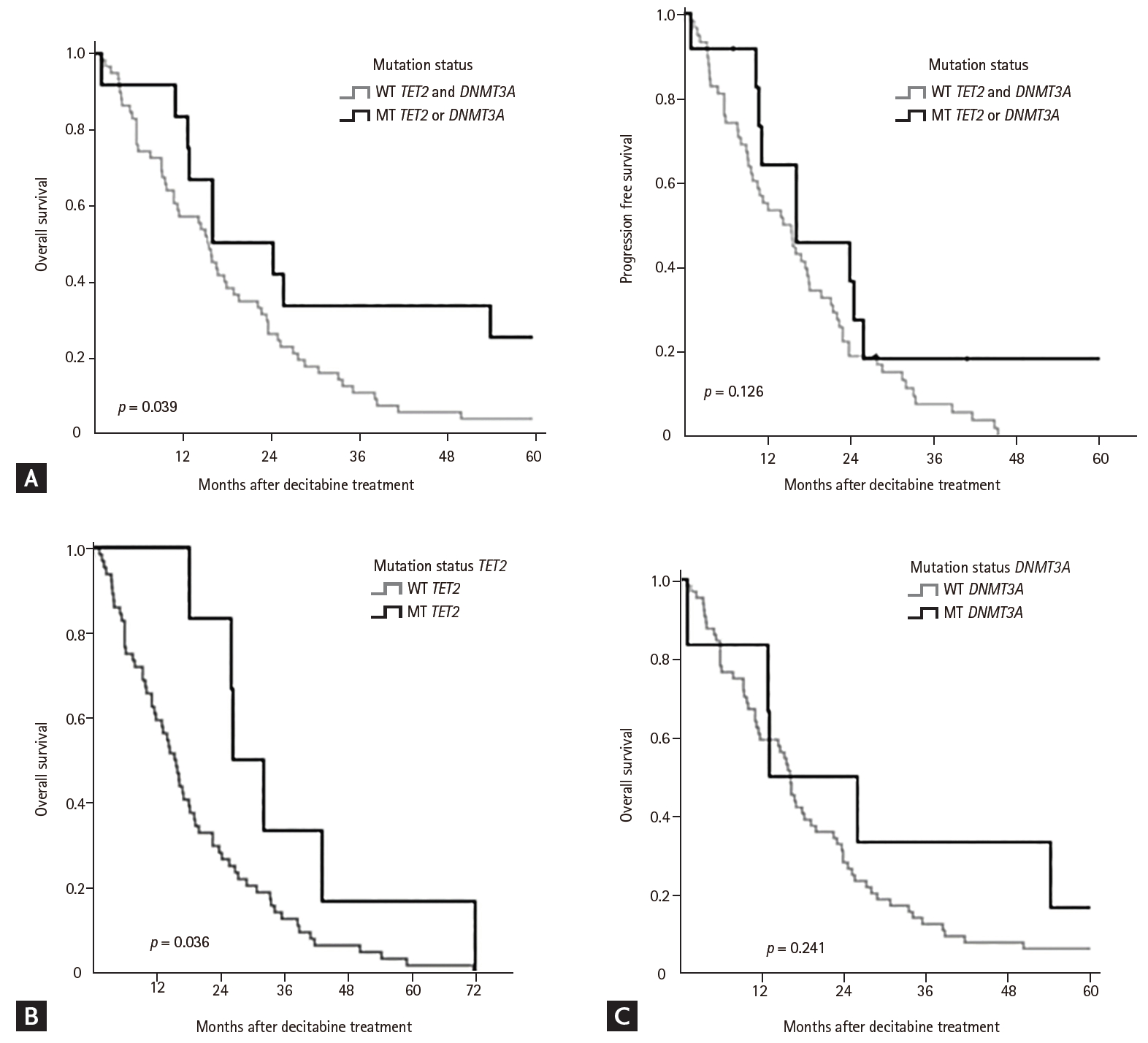
Figure 4.
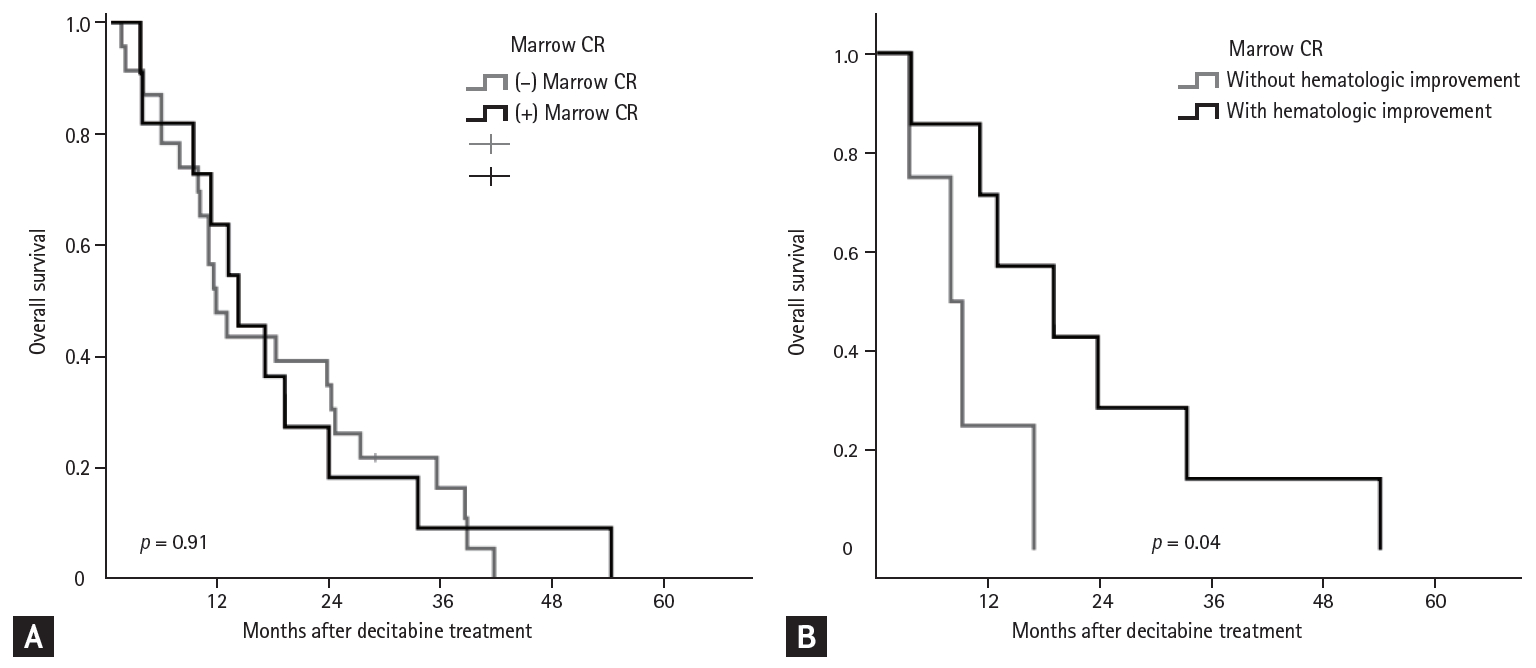
Table 1.
Values are presented as number (%).
TET2, ten-eleven-translocation 2; DNMT3A, DNA methyltransferase gene 3A; WHO, World Health Organization; RARS, refractory anemia with ring sideroblasts; MDS-U, myelodysplastic syndrome unclassifiable; RCMD, refractory cytopenia with multilineage dysplasia; RAEB, refractory anemia with excess blasts; CMML, chronic myelomonocytic leukemia; IPSS, International Prognostic Scoring System; INT, intermediate; R-IPSS, Revised International Prognostic Scoring System.
Table 2.
Table 3.
TET2, ten-eleven-translocation 2; DNMT3A, DNA methyltransferase gene 3A; MDS, myelodysplastic syndrome; BM, bone marrow; RAEB, refractory anemia with excess blasts; m-CR, marrow complete response; HI, hematologic improvement; CMML, chronic myelomonocytic leukemia; PD, progressive disease; RCMD, refractory cytopenia with multilineage dysplasia; CR, complete response; SD, stable disease; PR, partial response.
Table 4.
Values are presented as number (%).
MDS, myelodysplastic syndromes; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; RARS, refractory anemia with ring sideroblasts; MDS-U, myelodysplastic syndrome unclassifiable; RCMD, refractory cytopenia with multilineage dysplasia; RAEB, refractory anemia with excess blasts; CMML, chronic myelomonocytic leukemia; IPSS, International Prognostic Scoring System; INT, intermediate; R-IPSS, Revised International Prognostic Scoring System.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print



