INTRODUCTION
Asthma is a chronic airway disease characterized by airway inflammation, airway remodelling, and airway hyperresponsiveness (AHR) [1]. Airway remodelling is evident as structural changes in the airway, as represented by smooth muscle hyperplasia, mucous metaplasia, and subepithelial fibrosis in the histological evaluation of samples from lungs with chronic asthma [2,3]. Accumulating evidence suggests that airway remodelling is associated with the asthma-related progressive loss of lung function and is therefore linked to the severity of the disease [4]. However, airway remodelling is a complex process with multiple mechanisms that may have diverse effects on airway function, and the underlying pathways and the effective therapy to prevent asthma remain to be clarified.
MicroRNA (miRNA or miR) comprises non-coding RNAs of ~22 nucleotides that regulate gene expression via translational inhibition and destabilization of mRNA and helps to co-ordinate a variety of biological processes [5]. miR-21 is a crucial member of the miRNA family, and previous studies have confirmed that miR-21 expression is increased in allergic airway inflammation in mice [6,7] and in patients with bronchial asthma [8]. A recent study reported that miR-21 promotes steroid insensitivity and the severity of asthma in an animal model through suppression of histone deacetylase [9]. miR-21 can also promote type 2 helper T-cell (TH2) responses by two different pathways in allergic diseases. First, miR-21 can repress interleukin 12 (IL-12) gene expression, thus inhibiting TH1 responses and favouring TH2 responses [6,7]. Second, miR-21 can directly promote TH2 differentiation in a T-cell intrinsic manner by increasing Gata-3 and IL-4 expression early after T-cell activation [10]. Lu et al. [11] reported that miR-21 can modulate the proliferation and differentiation of eosinophils by reducing eosinophil count in miR-21-deficient mice.
Transforming growth factor ╬▓1 (TGF-╬▓1) is a cytokine whose level is elevated in the airway of patients with asthma [12,13]. This cytokine plays a role in airway remodelling by promoting collagen synthesis and inducing the expression of ╬▒-smooth muscle actin (╬▒-SMA) [14]. Canonical TGF-╬▓1 signalling phosphorylates Smad2 and Smad3 transcription factors. After associating with Smad4, the phosphorylated Smad complex translocates to the nucleus to promote the transcriptional target genes [15]. Smad7 plays an essential role in the negative-feedback regulation of TGF-╬▓1 signalling by preventing the phosphorylation of Smad2/3 [16]. Smad7 is a direct target of miR-21 [17]. In some studies of fibrotic diseases, the miR-21 and TGF-╬▓ŌĆōSmad pathways were both modulated to induce the epithelialŌĆōmesenchymal transition and progress to fibrosis [18]. However, few studies have evaluated the interplay between miR-21, Samd7, and airway remodelling, or have focused on smooth muscle cells, a key modulator of the asthma process.
In this study, we assessed the roles of miR-21 and Smad7 in the pathogenesis of chronic allergic asthma. We first examined the effects of miR-21 inhibition and its relationship with TGF-╬▓ŌĆōSmad signalling in a mouse model of ovalbumin (OVA)-induced chronic allergic asthma. We then explored the roles of miR-21 and Smad7 in TGF-╬▓1-induced human bronchial smooth muscle cells (hBSMCs). We found that miR-21 inhibition attenuated airway remodelling in chronic asthma by activating Smad7 expression in the animal model as well as in the in vitro study of hBSMCs. We describe here an miR-21ŌĆōTGF-╬▓1ŌĆōSmad7 axis, which appears to be involved in airway remodelling, and we identify miR-21 as a potential novel therapeutic target in patients with allergic asthma.
METHODS
Mice
Female BALB/c mice (Orient, Seongnam, Korea), 6 weeks of age, were used in all experiments. The mice were randomly allocated to the following groups: (1) control (CON); (2) ovalbumin (OVA) challenge; (3) OVA challenge + miRNA negative control (NC) as a vehicle control (OVA + miRNA NC); and (4) OVA challenge + miR-21 inhibitor (OVA + miR-21 inhibitor). All procedures were in accordance with the Laboratory Animals Welfare Act, Guide for the Care and Use of Laboratory Animals, and Guidelines and Policies for Rodent Experiments provided by the Institutional Animal Care and Use Committee (IACUC) of the College of Medicine of The Catholic University of Korea (No. CUMS-2016-0166-02). The IACUC and Department of Laboratory Animals of the Catholic University of Korea, Songeui Campus, was accredited as a Korea Excellence Animal Laboratory Facility by the Korea Food and Drug Administration in 2017 and acquired full accreditation by the Association for Assessment and Accreditation of Laboratory Animal Care International in 2018.
Protocol for sensitization and antigen challenge
The mice were immunized with 25 ╬╝g of OVA (chicken egg albumin, grade V, from Sigma-Aldrich, St. Louis, MO, USA) with 1 mg of aluminium hydroxide (alum) in 200 ╬╝L of phosphate-buffered saline (PBS). The mice were immunized by subcutaneous injection on days 0, 7, 14, and 21, and then challenged with intranasal OVA (20 ╬╝g/50 ╬╝L PBS) on day 33, 35, and 37 and then again twice a week for 3 months while anaesthetized with isoflurane (Vedco, St. Joseph, MO, USA). CON mice were treated with PBS in the same manner. Mice were killed 24 hours after the final OVA challenge, and bronchoalveolar lavage fluid (BALF) and lung tissues were obtained.
Administration of miR-21 inhibitor
We used the mirVana miR-21 inhibitor (Ambion, Applied Biosystems, Foster City, CA, USA) to inhibit the expression of miR-21 in this OVA-induced chronic asthma mouse model [19]. The mirVana miRNA inhibitor was used for the NC. The miR-21 inhibitor or miRNA NC (50 ╬╝g/50 ╬╝L in PBS) was given intranasally once a week for sensitization and then twice a week for 3 months, as shown in Fig. 1.
Measurement of AHR
AHR was measured as airway resistance (resistance of the respiratory system [RrS]) after methacholine administration and was recorded using the FlexiVent system (SCIREQ, Montreal, QC, Canada) [20]. Mice were anaesthetized with an intraperitoneal injection of a mixture of Rompun and Zoletil (1:4). The trachea was exposed and cannulated, and the animal was connected to a computer-controlled small-animal ventilator and ventilated with the following settings to achieve a mean lung volume close to that during spontaneous breathing: tidal volume of 10 mL/kg, frequency of 150 breath/minute, and positive end-expiratory pressure of 2 cmH2O. Mice were first exposed to nebulized PBS for 3 minutes to establish the baseline Rrs values and then to increasing concentrations of nebulized methacholine (6.25, 12.5, 25, and 50 mg/mL; Sigma-Aldrich) in PBS using an ultrasonic nebulizer (Aerosonic, DeVilbiss, Somerset, PA, USA). Recordings were taken for 3 minutes following each exposure. The Rrs values measured during each 3 minutes interval were averaged and are presented for each methacholine concentration.
Bronchoalveolar lavage
Bronchoalveolar lavage was performed immediately after the measurement of AHR. The exposed trachea was cannulated with silicone tubing attached to a 23-gauge needle on a 1 mL tuberculin syringe. BALF was collected after instillation of 0.8 mL of sterile PBS through the trachea into the lungs. The total cell counts in BALF were obtained using a LUNA Automated Cell Counter (Logos Biosystems, Inc., Annandale, VA, USA). BALF was centrifuged onto microscope slides at 2,000 rpm for 7 minutes in a Cytospin centrifuge (Thermo Fisher Scientific, Waltham, MA, USA) and stained with Diff-Quick (Sysmex, Kobe, Japan). The percentages of macrophages, eosinophils, lymphocytes, and neutrophils in BALF were obtained by counting 500 leukocytes in randomly selected fields under light microscopy.
Enzyme-linked immunosorbent assay
The concentrations of IL-4, IL-5, IL-13, and interferon ╬│ (IFN-╬│) in BALF were measured using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturerŌĆÖs instructions. Active TGF-╬▓1 concentration was measured after pretreating the samples with 10 ╬╝L of 1 N hydrochloric acid (HCl) for 10 minutes at room temperature to convert the active TGF-╬▓1 and then neutralizing the samples with 1.2 N NaOH/0.5 M hydroxyethyl piperazine ethane sulfonicacid (HEPES) according to the manufacturerŌĆÖs instructions (R&D Systems).
H&E and periodic acid-Schiff staining
Lung samples were fixed in 4% paraformaldehyde for 24 hours and then embedded in paraffin wax. Sections were cut at a thickness of 4 ╬╝m using a microtome, and deparaffinized tissue sections were stained with H&E for the examination of airway inflammation. For assessment of airway inflammation, the slides were numbered randomly and evaluated independently by two blinded investigators. The quantity of perivascular or peribronchial inflammation was assessed as described previously [21]. The following 5-point scoring system (grades 0 to 4) was used: no inflammation (grade 0), occasional cuffing with inflammatory cells (grade 1), most bronchi or vessels surrounded by a thin layer (grade 2), moderate layer (grade 3), or thick layer (grade 4) of inflammatory cells. Periodic acid-Schiff (PAS) staining was used to identify goblet cell hyperplasia and submucosal gland hypertrophy. Hyperplasia of goblet cells in the epithelial lining is expressed as the percentage of the goblet cells in the epithelial cells. A 5-point scoring system (grades 0 to 4) was used to quantify the percentages of goblet cells exhibiting hyperplasia as follows: no goblet cells (grade 0), < 25% (grade 1), 25% to 50% (grade 2), 51% to 75% (grade 3), and > 75% (grade 4).
Immunohistochemistry
Six-micrometre-thick sections of lung tissue from each paraffin block were deparaffinized with xylene and hydrated in ethanol. For immunohistochemical detection of collagen III, collagen V, and ╬▒-SMA, the lung sections were incubated overnight at 4┬░C with anti-collagen III antibody, anti-collagen V antibody (both from Abcam, Cambridge, MA, USA), and anti-╬▒-SMA antibody (Dako, High Wycombe, UK). Immunoreactivity was detected by sequential incubation of lung sections with a biotinylated secondary antibody followed by peroxidase reagent (Vector Laboratories, Burlingame, CA, USA) and diaminobenzidine chromogen (Invitrogen, Carlsbad, CA, USA). The results are expressed as the area of immunostaining per micrometre length of the basement membrane of bronchioles with an internal diameter of 150 to 200 ╬╝m. At least 10 bronchioles were counted in each slide using a slide scanner (Pannoramic MIDI, 3DHISTECH Ltd., Budapest, Hungary).
Hydroxyproline colorimetric assay
Hydroxyproline content was quantified in 60 mg of lung tissue from each mouse to determine the total amount of collagen. A hydroxyproline colorimetric assay kit (BioVision, Milpitas, CA, USA) was used according to the manufacturerŌĆÖs instructions.
Cell culture, transfection, and signal activation
hBSMCs were obtained from Lonza (Walkersville, MD, USA) and were maintained in smooth muscle basal medium supplemented with 5% (v/v) foetal bovine serum, 5 ng/mL insulin, 2 ng/mL human basic fibroblast growth factor, 50 ng/mL human epidermal growth factor, 50 ╬╝g/mL gentamicin, and 50 ng/mL amphotericin B at 37┬░C in 5% CO2. Cells between passages 3 and 8 were used for all experiments. We used the mirVana miR-21 inhibitor to inhibit the expression of miR-21 in hBSMCs. The Vana miRNA negative inhibitor was used as the NC. hBSMCs were grown in 100 mm culture dishes overnight, and cells at 60% to 70% confluence were transfected with 30 pmol of the miR-21 inhibitor or negative inhibitor using Lipofectamine 2000 (Invitrogen). After 72 hours of incubation, the cells were cultured under serum-free conditions for 16 hours and then treated with recombinant human TGF-╬▓1 (R&D Systems) for the indicated times.
Real-time polymerase chain reaction
Total RNA was isolated from lung homogenates using TRIzol (Invitrogen) and reverse-transcribed. Real-time polymerase chain reaction (PCR) was performed using a CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA). Reactions were amplified using the selective primers described in Table 1, and an iQ SYBR gene expression assay (Bio-Rad Laboratories), according to the manufacturerŌĆÖs instructions.
Western blot analysis
The lungs were sonicated in radioimmunoprecipitation assay (RIPA) buffer with ceramic beads at 5,000 rpm for 20 seconds. The lung homogenates were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted with monoclonal antibodies to the specific proteins, as indicated. Antibodies to Smad7 (#ab190987) and ╬▓-actin (#ab8227) were purchased from Abcam.
Statistical analysis
All data are presented as the mean ┬▒ SEM. Results were compared between groups using one-way analysis of variance (ANOVA) with the Tukey post hoc test or two-way ANOVA with the Bonferroni test. GraphPad Prism statistical software package (San Diego, CA, USA) was used for the analyses. A p value of < 0.05 was considered to be significant.
RESULTS
Establishment of the mouse model of OVA-induced chronic allergic asthma and administration of the miR-21 inhibitor
To investigate the effects of miR-21 inhibition on chronic allergic asthma, we used a well-established OVA-induced asthma mouse model [22]. Mice were sensitized to and challenged with OVA with and without the miR-21 inhibitor or miRNA NC, as described in Fig. 1 We first used TaqMan PCR analysis to evaluate the expression of miR-21 in this OVA-induced chronic allergic asthma mouse model. Compared with OVA + miRNA NC mice, miR-21 expression was increased by 10 to 15-fold in OVA-sensitized and -challenged mice and in miRNA NC-treated OVA (OVA + miRNA NC) mice. By contrast, miR-21 expression was decreased by threefold in OVA-administered mice treated with the miR-21 inhibitor (OVA + miR-21 inhibitor) (in Taq-Man RT-qPCR analysis section of Supplementary methods and Supplementary Fig. 1A).
A previous study showed that IL-12p35 is an miR-21 mRNA target [23] and is downregulated in asthma [6]. We next evaluated the expression of IL-12p35 in the OVA-induced chronic allergic asthma mouse model. As shown in Supplementary Fig. 1B, the mRNA levels of IL-12p35 were markedly reduced following OVA administration but were markedly increased following treatment with the miR-21 inhibitor. Collectively, these data indicate that the miR-21 inhibitor was effective in OVA-induced chronic allergic asthma mouse model.
Reduction in airway inflammation and AHR by miR-21 inhibition
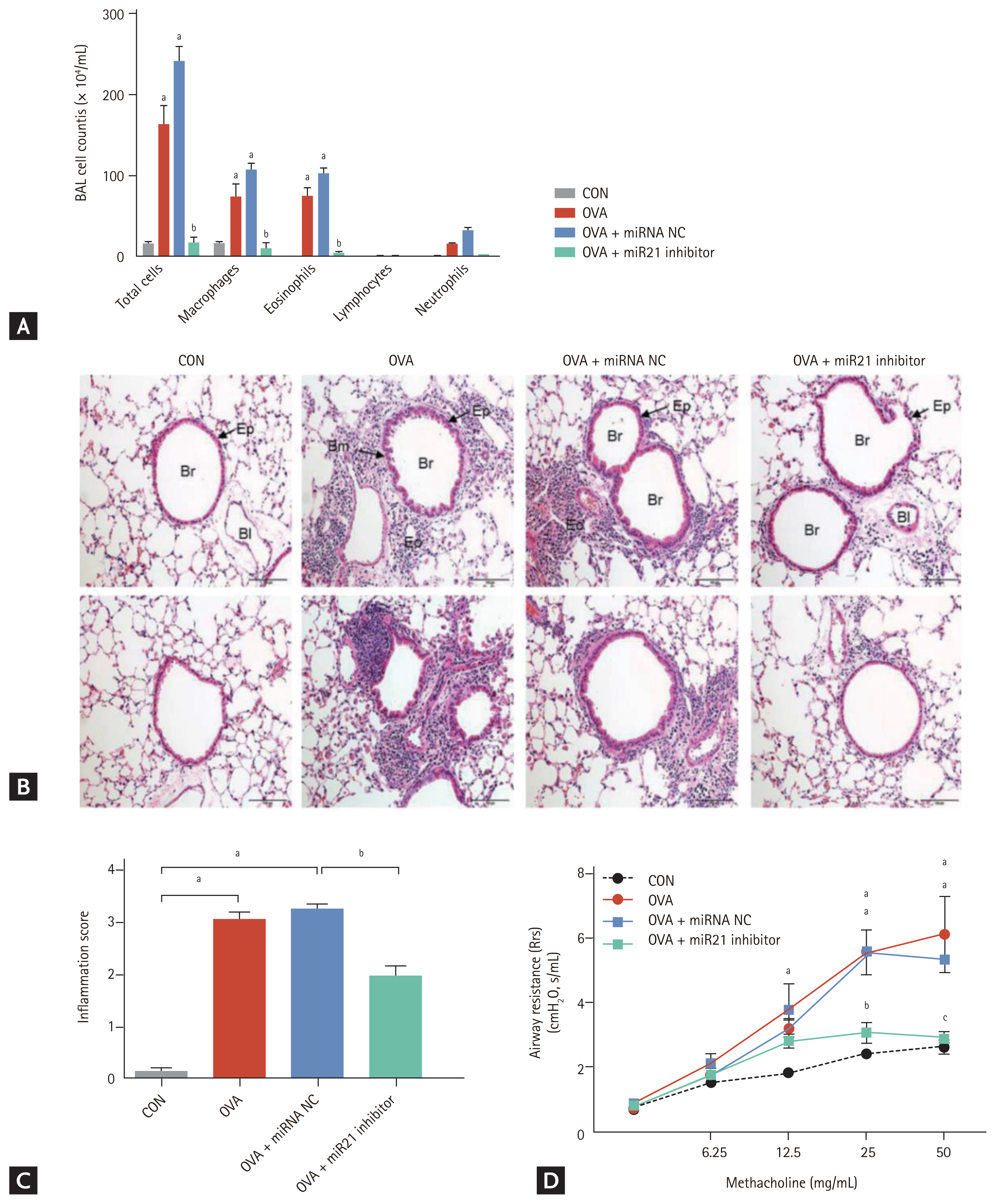
To determine the role of miR-21 inhibition on airway inflammation, we counted the total number of cells and the percentages of macrophages, lymphocytes, neutrophils, and eosinophils in BALF. The total number of cells and percentages of macrophages and eosinophils were significantly higher in the OVA and OVA + miRNA NC groups than in the CON group (p < 0.001). By contrast, treatment with miR-21 inhibitor significantly decreased the total cell count and percentage of eosinophils in BALF (p < 0.001) compared with the OVA + miRNA NC group (Fig. 2A). H&E-stained lung sections from OVA-administered and OVA + miRNA NC mice showed an increase in the infiltration of inflammatory cells in both the peribronchial and perivascular regions. However, miR-21 inhibitor treatment markedly reduced the infiltration of inflammatory cells in the airway, as evidenced by the lower absolute cell count in BALF (Fig. 2B and 2C).
Rrs was analysed to determine the effect of miR-21 inhibition on AHR. Rrs was elevated in OVA and OVA + miRNA NC mice but was markedly lower (p < 0.001) in the OVA-treated groups treated with the miR-21 inhibitor; this decrease exhibited a methacholine dose-dependent pattern (Fig. 2D).
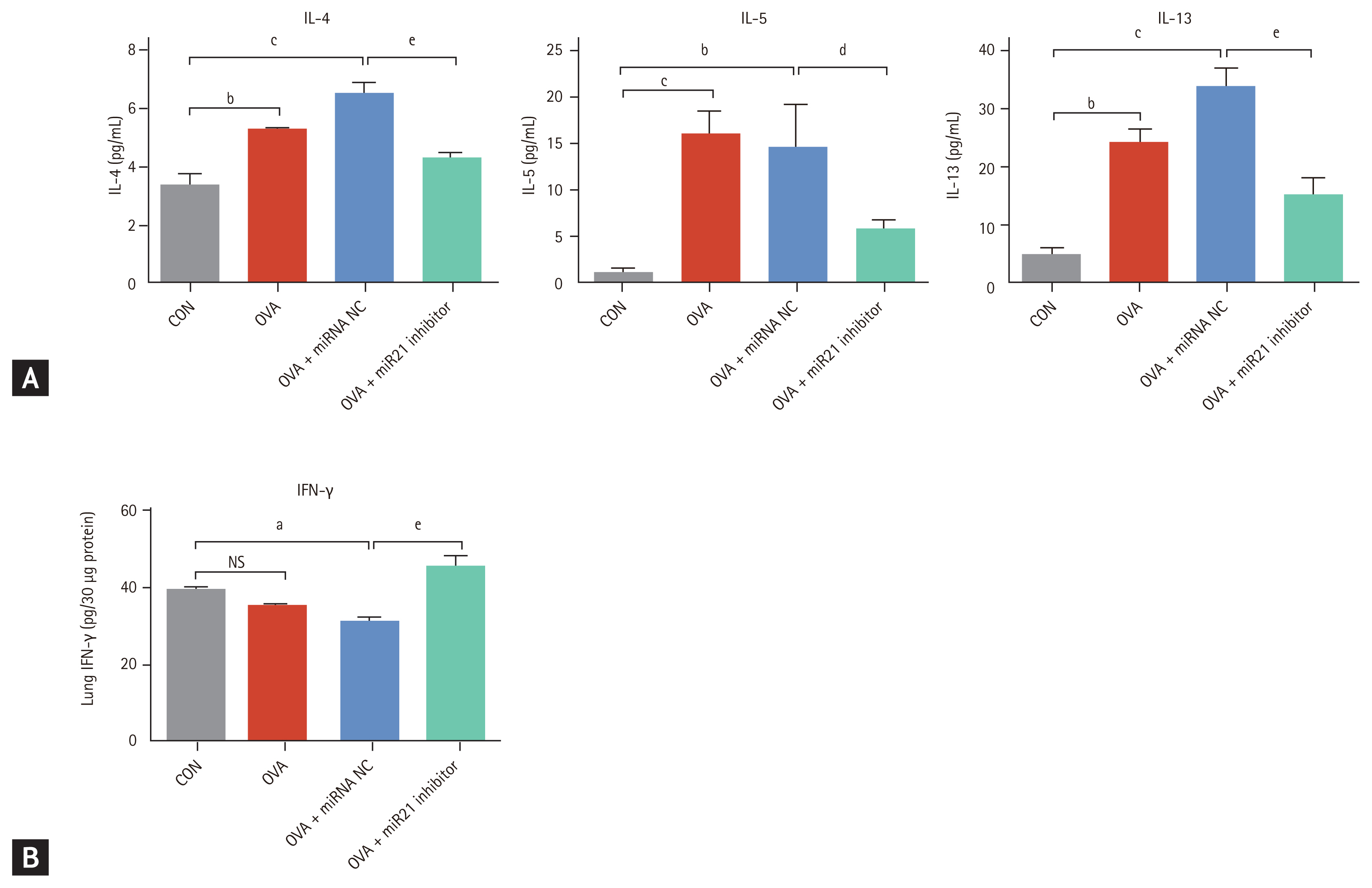
To investigate the effects of miR-21 inhibition on inflammatory cytokine expression, we measured the levels of TH2 and TH1 cytokines in the lungs of the four groups. OVA + miR-21 inhibitor mice exhibited significantly lower levels of the TH2 cytokines IL-4 (p < 0.001), IL-5 (p < 0.05), and IL-13 (p < 0.001) than OVA + miRNA NC mice (Fig. 3A). OVA + miR-21 inhibitor-treated mice also had significantly higher levels of IFN-╬│ (p < 0.001) than OVA + miRNA NC mice (Fig. 3B). Taken together, these findings suggest that miR-21 inhibition reduced airway inflammation and AHR in this OVA-induced chronic allergic asthma model.
Attenuation of airway remodelling by miR-21 inhibition
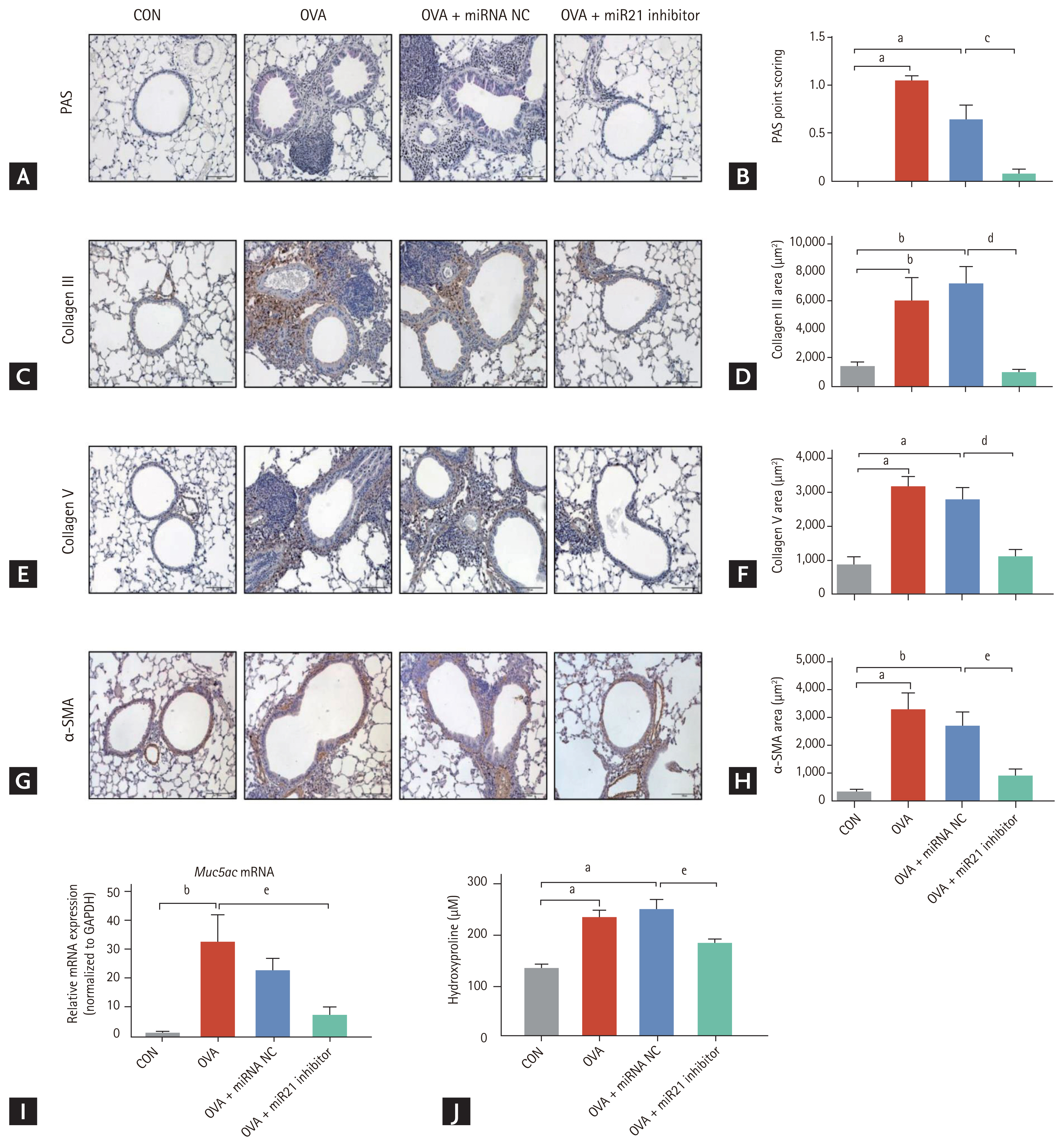
Airway remodelling is a critical structural alteration associated with chronic allergic asthma and is manifested as goblet cell hyperplasia, peribronchial subepithelial fibrosis, and smooth muscle cell hypertrophy. To examine the effects of miR-21 inhibition on these pathological components in OVA-induced chronic allergic asthma, we first examined mucus production in the lungs of these mice following OVA sensitization and challenge. OVA and OVA + miRNA NC mice exhibited more mucus-secreting cells than CON mice, as determined by PAS staining (Fig. 4A) and the hyperplasia score. miR-21 inhibitor-treated mice had significantly (p < 0.001) fewer hyperplastic goblet cells than OVA + miRNA NC mice (Fig. 4B). As shown in Fig. 4I, the mRNA levels of Muc5ac increased markedly in the OVA group but were much lower in the lung tissue following treatment with the miR-21 inhibitor.
To investigate the effects of miR-21 inhibition on peribronchial subepithelial fibrosis, we measured the levels of collagen III and V. Collagen III and V immunostaining of the peribronchial and perivascular regions was significantly greater in OVA and OVA + miRNA NC mice than in CON mice. Collagen immunostaining was significantly lower in samples from mice treated with the miR-21 inhibitor than OVA + miRNA NC mice (Fig. 4CŌĆō4F).
We measured hydroxyproline content to determine the total collagen level in the lungs of mice in the four groups. Hydroxyproline content was significantly (p < 0.05) lower in the miR-21 inhibitor-treated mice than in the OVA + miRNA NC mice (Fig. 4J). Lastly, we examined whether the miR-21 inhibitor acted on smooth muscle hyperplasia, one of the most important aspects of airway remodelling. OVA and OVA + miRNA NC mice showed increased peribronchial ╬▒-SMA immunostaining. By contrast, the expression of ╬▒-SMA was inhibited by the miR-21 inhibitor (Fig. 4G). This pattern was consistent with the quantification of the area of ╬▒-SMA staining (Fig. 4H). Collectively, these data indicate that the miR-21 inhibitor was effective in preventing or limiting airway remodelling.
Role of the TGF-╬▓ŌĆōSmad7 signalling pathway in miR-21 inhibition
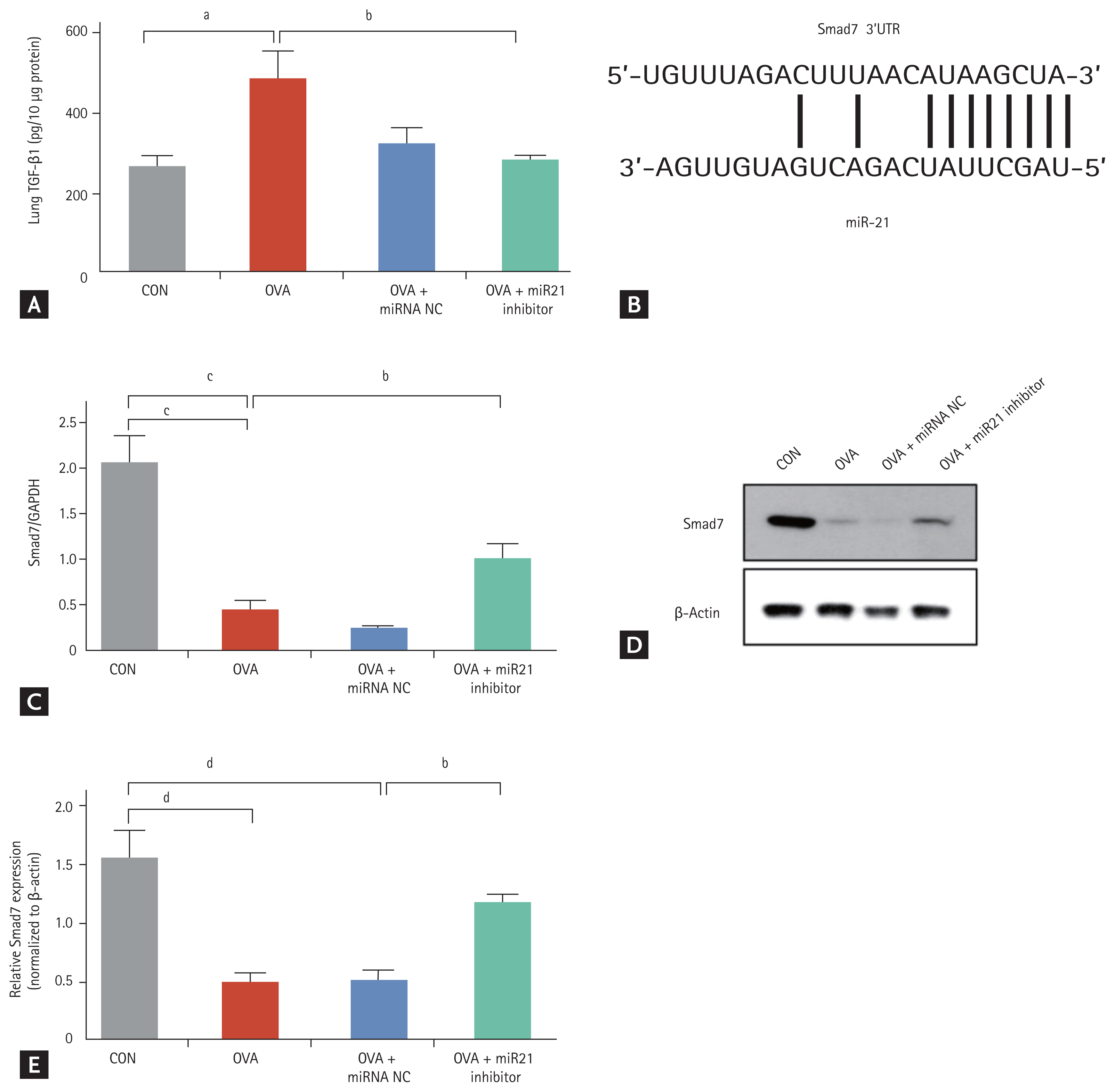
TGF-╬▓1 is directly involved in airway remodelling in asthma [14,24]. We wondered whether TGF-╬▓1 might also be involved in airway remodelling in this OVA-induced chronic asthma mouse model. The TGF-╬▓1 level was much higher in lung homogenates from OVA-challenged mice (p < 0.05) than from the CON mice. The TGF-╬▓1 level was significantly lower in lung homogenates from mice treated with the miR-21 inhibitor (p < 0.05) than from OVA-challenged mice (Fig. 5A).
Smad7 has been shown to inhibit the TGF-╬▓ signalling pathway [15,25] and to be a direct target of miR-21 in various diseases [26,27]. Therefore, to examine the suppression of Smad7 by miR-21, we used TargetScan and miRanda to predict the paired target regions of the Smad7 3ŌĆ▓untranslated region and the miR-21 seed sequence (Fig. 5B). We first checked to determine whether miR-21 inhibitor treatment induced Smad7 expression in this model of OVA-induced chronic allergic asthma. expression was significantly reduced in OVA-challenged mice (p < 0.001) and was increased by miR-21 inhibitor treatment compared with OVA + miRNA NC treatment (Fig. 5C). Smad7 protein expression was increased by 2 to 3-fold in mice treated with the miR-21 inhibitor compared with the OVA + miRNA NC mice (Fig. 5D and 5E). These results suggest that miR-21 inhibition can inhibit TGF-╬▓1 expression and upregulate Smad7 gene and protein expression.
Effects of miR-21 inhibition on the collagen production and pro-fibrotic gene expression in TGF-╬▓1-induced hBSMCs
To investigate whether miR-21 inhibition influences Smad7 expression, we examined the expression of Smad7 protein in TGF-╬▓1-activated hBSMCs. The content of Smad7 protein was lower after TGF-╬▓1 stimulation than with no treatment. The miR-21 inhibitor increased Smad7 protein level (Fig. 6A). Altered extracellular matrix deposition is an important contributor to the remodelling of airways in chronic allergic asthma. We next examined whether miR-21 inhibition affects collagen production in hBSMCs. TGF-╬▓1 stimulation increased the production of fibrotic matrix components such as collagen types I (COL1A1), IV (COL4A1), and VII (COL7A1) in hBSMCs. These changes were attenuated substantially by treatment with the miR-21 inhibitor (Fig. 6B). Expression of COL3A1 was not changed.
These findings prompted us to explore further the mechanisms underlying the anti-fibrotic effects of miR-21 inhibition in hBSMCs. The mRNA levels of plasminogen activator inhibitor-1 (PAI-1) and connective tissue growth factor (CTGF) increased markedly after treatment with TGF-╬▓1 and decreased after treatment with the miR-21 inhibitor compared with miRNA NC treatment (both comparisons, p < 0.001) (Fig. 6C). As expected, treatment of hBSMCs with the miR-21 inhibitor reversed the TGF-╬▓1-induced profibrotic gene expression and collagen production. We further examined the role of miR-21 inhibition in the expression of ╬▒-SMA (ACTA2) as a marker of bronchial smooth muscle hypertrophy in hBSMCs. Consistent with the above results, the miR-21 inhibitor also reduced ACTA2 mRNA expression (Fig. 6D). Taken together, these data provide in vitro evidence that miR-21 inhibition decreases airway remodelling by modulating TGF-╬▓1ŌĆōSmad7 signalling.
DISCUSSION
Asthma is a complex disease that is characterized by airway inflammation and remodelling, which are associated with an irreversible loss in lung function [4,28]. However, no treatment can reverse the airway remodelling. In this study, we found that miR-21 inhibition attenuated airway inflammation and airway remodelling in this mouse model of OVA-induced chronic allergic asthma. Structural changes such as submucosal gland hyperplasia, extracellular matrix deposition, and smooth muscle hyperplasia were attenuated markedly by the miR-21 inhibitor in mice with OVA-induced asthma. These anti-remodelling effects of the inhibitor were verified by an in vitro study using hBSMCs, which showed that miR-21 is a key regulator of airway remodelling.
Other miRNA such as miR-126 or miR-145 have also been studied to investigate their possible therapeutic effects in asthma [29]. Shi et al. [30] reported that overexpression of miR-155-5p induced the proliferation and migration of bronchial smooth muscle cells after stimulation with IL-13. Mattes et al. [31] reported that the selective blockade of miR-126 by antagomir suppressed the asthmatic phenotype in a house dust mite-induced acute asthma model. However, the same group reported finding in a chronic asthma model that inhibition of miR-126 by antagomir did not have any effects on airway inflammation or remodelling, which suggested a temporal difference in the role of miR-126 in regulating allergic airway disease [32]. By contrast, in the current study and in our earlier study [19], we found that suppression of miR-21 had protective effects against the pathogenesis of asthma, irrespective of the state of the disease process, in both acute and chronic models.
Some studies of the inflammatory process have reported that miR-21 has detrimental effects on asthma through TH1 polarization, TH2 differentiation, and eosinophil proliferation [6,7,11]. It is accepted that miR-21 affects the equilibrium between TH1 and TH2 by modulating IL-12p35, the main target of miR-21, and that this process is involved in the airway inflammation associated with asthma. In the current study, the decreased expression of IL-12p35 in the OVA group was reversed in the miR-21 inhibitor-treated group. Changes in parameters related to airway inflammation, including total cell and eosinophil counts in BALF and peribronchial infiltration in tissue, were also attenuated by miR-21 inhibitor. In addition, the concentrations of TH2 cytokines IL-4, IL-5, and IL-13 decreased whereas the concentration of the typical TH1 cytokine IFN-╬│ increased in the miR-21 inhibitor-treated mice. Collectively, these results support the beneficial effect of miR-21 inhibition on airway inflammation in a chronic asthma model.
Recent studies of the role of miR-21 in airway remodelling have reported that miR-21 regulates the proliferation of airway smooth muscle cells and fibroblasts, two major contributors to the structural alterations, through various signal pathways [33ŌĆō35]. In the present study of hBSMCs, we found that the miR-21 inhibitor reduced the expression of mRNAs for TGF-╬▓1-inducible factors related to airway remodelling including collagen genes and profibrotic genes. These data provide in vitro evidence for the anti-remodelling action of miR-21 inhibition in hBSMCs. We also confirmed the anti-remodelling effect by controlling miR-21 at the lung tissue level in the mouse asthma model.
TGF-╬▓1 is a well-known critical mediator of airway remodelling in the asthmatic lung and that its actions influence peribronchial fibrosis and airway smooth muscle proliferation [14,22,36,37]. TGF-╬▓1 is tightly regulated in both positive and negative manners. One of the important negative-feedback regulations is via induction of Smad7 expression [25]. On the other hand, Smad7 is known to target gene of miR-21 [17]. In this study, we found markedly reduced TGF-╬▓1 protein content in the lungs of mice treated with the miR-21 inhibitor compared with OVA mice. This downregulation of Smad7 at the mRNA and protein levels in OVA-induced chronic allergic asthma was inhibited by the miR-21 inhibitor. Moreover, the TGF-╬▓1-stimulated inactivation of Smad7 was reversed by the miR-21 inhibitor in hBSMCs, which implies a regulatory role of miR-21 in TGF-╬▓1ŌĆōSmad7 signalling.
In the pathogenesis of asthma, phosphatase and tensin homologue (PTEN) and its downstream PI3KŌĆōAkt pathway have been investigated more as the target signals of miR-21 than TGF-╬▓1ŌĆōSmad7 signalling [9,34]. We examined PTEN signalling and did not observe upregulated expression of PTEN mRNA after administration of miR-21 inhibitor in this OVA-induced chronic asthma mouse model (data not shown). Interaction between miR-21 and TGF-╬▓1ŌĆōSmad signalling occurs in fibrotic diseases such as hepatic or renal fibrosis [38]. Our findings are consistent with the findings of recent in vitro studies that reported that miR-21 promotes airway remodelling through regulation of the TGF-╬▓ŌĆōSmad pathway. In particular, Yu et al. [33] reported that TGF-╬▓ could induce miR-21 expression and aggravate airway remodelling in human bronchial fibroblasts. Taken together, these data suggest a mutual regulation between miR-21 and the TGF-╬▓ŌĆōSmad pathway [35].
We observed that TGF-╬▓1 stimulated the expression of mRNA for collagens, profibrotic genes, and ACTA2, all of which are crucial components of airway remodelling, in hBSMCs. Previous studies have reported that many actions of TGF-╬▓1 on airway smooth muscle cells are mediated through the induction of collagens I and III [39] and CTGF [40] in tissues from asthmatic patients and in hBSMCs. Similar to previous studies, our study also showed that TGF-╬▓1 induced mRNA expression related to genes for collagen deposition and that miR-21 inhibition may reduce collagen deposition induced by PAI-1 and CTGF through the TGF-╬▓1ŌĆōSmad7 signalling pathway. We also found that TGF-╬▓1 induced the expression of profibrotic genes and ╬▒-SMA, which promote the activation of bronchial smooth muscle cells and airway remodelling. We found that the airway remodelling changes observed in hBSMCs were significantly reduced by the miR-21 inhibitor.
In the past few years, miR-21 has emerged as a novel therapeutic target for the treatment of asthma [6ŌĆō9,34]. However, the role of miR-21 in airway remodelling has not been elucidated completely. In this study, we determined, for the first time, that miR-21 inhibition affects airway remodelling through the miR-21ŌĆōTGF-╬▓1ŌĆōSmad7 axis in a chronic allergic asthma mouse model and in hBSMCs. These findings suggest that miR-21 inhibitors may be useful for preventing airway remodelling in allergic asthma patients.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print





