 |
 |
|
|
|
See editorial "Simple renal cysts: no longer simple?" on page 283.
Abstract
Background/Aims
The prevalence of simple renal cysts increases with age; however, they are occasionally found in adults aged < 40 years. This cross-sectional study evaluated the clinical significance of simple cysts in young adults, focusing on their associations with hematuria and albuminuria.
Methods
Adults aged < 40 years who underwent comprehensive medical examination between January 2005 and December 2013 were included. Simple renal cysts were identified by ultrasonography.
Results
Renal cysts were found in 276 of the 5,832 subjects (4.7%). Subjects with medullary sponge kidney (n = 1) or polycystic kidney disease (n = 5) were excluded. A single cyst and multiple cysts were found in 234 (4.0%) and 42 (0.7%) subjects, respectively. Age, high systolic blood pressure, and history of hypertension were independent risk factors for the presence of simple cysts. Simple cysts were not associated with an increased prevalence of hematuria. However, subjects with cysts showed a higher prevalence of albuminuria than those without (11.3% vs. 4.5%, p < 0.001). Multivariate analysis revealed that the existence of simple renal cysts was associated with a 2.30-fold increased prevalence of albuminuria (95% confidence interval, 1.512 to 3.519; p < 0.001) independent of other risk factors.
A simple renal cyst consists of a thin wall with a single-layer of epithelium [1]. Regular follow-up is not recommended, because these cysts are usually asymptomatic and have low malignant potential [2]. The prevalence of simple renal cysts is three times higher in men than in women [3,4]. Moreover, its prevalence increases with age, ranging from 6% in adults aged from 21 to 40 years to 35% in those aged > 70 years [3]. The pathogenesis of simple renal cysts is unclear. One hypothesis is that renal parenchymal ischemia causes compensatory hyperfiltration and tubular hypertrophy, resulting in cyst formation [5].
There is a growing body of evidence showing that simple renal cysts are associated with hypertension [6ŌĆō11]. Several observational studies have reported that the incidence and prevalence of hypertension are higher in patients with simple renal cysts [6,7]. Additionally, the adjusted odds ratio (OR) for the risk of pre-hypertension and hypertension revealed that the presence of simple renal cysts is independently associated with high blood pressure (BP) [9]. A study from South Korea has demonstrated that the effect of simple cysts on hypertension is more apparent in men and elderly people, especially those > 60 years of age [6]. However, data on the relationship between simple renal cysts and renal parenchymal disease or renal dysfunction have been conflicting [12]. Chang et al. [3] and Al-Said et al. [13] have shown a positive correlation between simple renal cysts and renal dysfunction.
Based on previous studies, we evaluated the association between simple renal cysts and markers of renal parenchymal injury, such as hematuria and albuminuria. We included only young adults aged < 40 years to minimize the effects of the aging process.
For this cross-sectional study, we obtained the patientsŌĆÖ information and clinical data from the health promotion center of Samsung Medical Center. The study subjects were adults younger than 40 years who underwent a comprehensive medical examination between January 2005 and December 2013. A total of 5,838 subjects were included in the study. We excluded foreigners or subjects for whom we who did not have data on kidney ultrasonography, urine albumin-to-creatinine ratio (uACR), BP, and serum creatinine level. We further excluded six patients with cystic kidney disease (five with autosomal dominant polycystic kidney disease [ADPKD] and one with medullary sponge kidney). We regarded ADPKD if the patients were consulted to nephrologists and were confirmed based on family history and typical ultrasonography findings of polycystic kidney.
The subjects completed structured questionnaires about comorbidities (e.g., diabetes mellitus and hypertension), smoking habits, and drug usage. The presence of hypertension was defined as the current use of hypertensive medication or a history of hypertension. Diabetes mellitus was defined as the current use of an oral hypoglycemic agent or insulin, or hemoglobin A1c (HbA1c) Ōēź 6.5%. We categorized the subjects into three groups according to smoking status: current smokers, ex-smokers, and never smokers. Clinical dataŌĆöincluding age, sex, height, weight, abdominal girth, systolic BP, and diastolic BPŌĆöwere extracted from electronic medical records. Using body weight and height data, we calculated the body mass index (BMI) as body weight (kg)/height2 (m2). A BMI of 23 to 24.9 kg/m2 was classified as overweight and Ōēź 25 kg/m2 as obese, according to the World Health Organization criteria for Asian and Pacific populations.
Laboratory dataŌĆöincluding serum creatinine, uric acid, total CO2, HbA1c, fasting glucose, serum triglyceride, serum high-density lipoprotein cholesterol (HDL-C), serum low-density lipoprotein cholesterol (LDL-C), findings of urine microscopy for hematuria, and uACRŌĆöwere also collected. Serum creatinine was measured using the Jaffe method during the study period. Hematuria was defined as the presence of Ōēź 5 red blood cells per high-power field in a urine specimen.
This study was approved by the Institutional Review Board of the Samsung Medical Center (2017-12-013-001). Informed consent was not required due to the retrospective design of this study.
Albuminuria was defined as a uACR of Ōēź 30 ╬╝g/mg and divided into microalbuminuria (< 299 ╬╝g/mg) and overt proteinuria (Ōēź 300 ╬╝g/mg). We used the Chronic Kidney Disease Epidemiology Collaboration equation to calculate the estimated glomerular filtration rate (eGFR) for assessing kidney function.
At the time of the health examination, a radiology specialist performed the kidney ultrasonography and confirmed the presence and number of simple renal cysts. We reviewed the reports and images of kidney ultrasonography. The diagnostic criteria for simple renal cysts were round or oval cysts with thin, smooth, and sharply marginated walls with no evidence of calcification or malignancy. The characteristics of the simple renal cysts (e.g., number and location) were also examined.
Categorical variables and continuous variables not following a normal distribution were expressed as number (percentage) and median (interquartile range), respectively. The group differences for continuous variables between the control group and the simple cyst group were determined using the Mann-Whitney test. The chi-square test was used for comparing categorical variables. We used the Kruskal-Wallis test to compare > 2 groups wherever appropriate. Univariate and multivariate logistic regression analyses were performed to identify factors associated with the presence of simple renal cysts. We conducted the logistic analyses of albuminuria in the same way. Multivariate analysis was performed using variables that were significant in the univariate analysis and believed to affect the dependent variable. Additionally, we performed 1:2 propensity score matching analysis to confirm the association between simple cysts and albuminuria in a balanced covariate distribution. The variables used for matching were age, male sex, BMI, systolic BP, hypertension, diabetes mellitus, eGFR, history of smoking, and serum uric acid levels. Standardized mean differences were used to compare baseline characteristics between propensity-matched groups, and < 0.1 was defined as a meaningful balance.
A p value of < 0.05 was considered to represent statistical significance. We used SPSS Statistics version 24 (IBM Corp., Armonk, NY, USA) to perform all statistical analyses.
We analyzed 5,832 subjects in this study. The overall prevalence of simple renal cysts in young adults was 4.7%. A single cyst and multiple cysts (Ōēź 2) were found in 234 (4.0%) and 42 (0.7%) subjects, respectively. The locations of the single cyst were the cortex in 187, medulla in 26, and parapelvic region in 21 subjects. Table 1 shows the baseline characteristics of the control group (n = 5,556) and the cyst group (n = 276). The proportion of men was higher in the cyst group than in the control group (60.5% vs. 46.5%, p < 0.001). The cyst group showed higher BMI (23.2 kg/m2 vs. 22.4 kg/m2, p = 0.001), larger abdominal girth (82 cm vs. 80 cm, p = 0.001), higher systolic/diastolic BP (118/74 mmHg vs. 113/70 mmHg, p < 0.001), higher prevalence of hypertension (13.4% vs. 5.7%, p < 0.001), and higher proportion of current smokers (37.9% vs. 30.3%, p = 0.027) than the control group. The serum triglyceride level was higher (100 mg/dL vs. 88 mg/dL, p = 0.003) and the serum HDL-C level was lower (53 mg/dL vs. 57 mg/dL, p = 0.001) in the cyst group than in the control group. The serum uric acid level was also higher in the cyst group than in the control group (5.5 mg/dL vs. 4.9 mg/dL, p < 0.001).
Table 2 presents the results of univariate and multivariate logistic regression analyses of simple renal cysts. In the univariate analyses, there were a few variables associated with presence of renal cysts: older age, male sex, higher BMI, larger abdominal girth, higher systolic and diastolic BPs, history of hypertension, current smoking, lower HDL-C level, and higher uric acid level. As BMI and abdominal girth have a strong linear correlation, BMI was included in the multivariate analysis instead of abdominal girth. Multivariate analyses of simple renal cysts revealed that age (OR, 1.07; 95% confidence interval [CI], 1.02 to 1.12 per 1-year increment; p = 0.002), systolic BP (OR, 1.01; 95% CI, 1.00 to 1.02 per 1-mmHg increment; p = 0.006), and hypertension (OR, 1.85; 95% CI, 1.24 to 2.76; p = 0.003) were independent predictors for the existence of simple cysts.
Additionally, we performed interaction term analyses between variables associated with the presence of simple cysts: age, BMI, systolic BP, history of hypertension, and male sex. A significant interaction between hypertension and male sex was observed (p = 0.004). Next, stratified analyses were performed, in which the subjects were divided into two groups according to the presence of hypertension. This showed that male sex was associated with increased odds of simple cysts only in subjects without hypertension (OR, 1.786; 95% CI, 1.373 to 2.323; p < 0.001). Whereas, male sex was not a risk factor for simple cysts in subjects with hypertension (OR, 0.528; 95% CI, 0.241 to 1.156; p = 0.112).
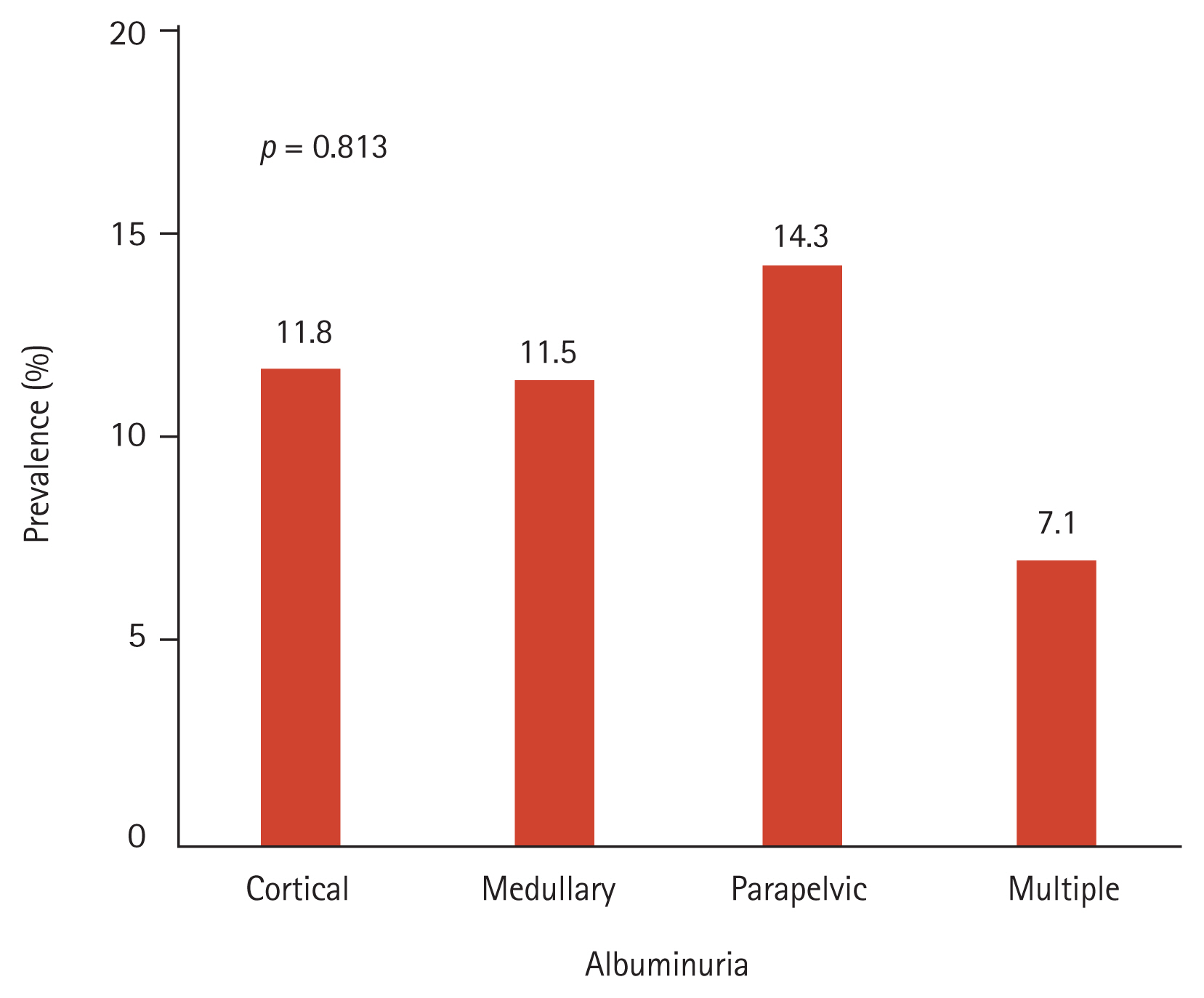
The prevalence of hematuria was not different between the control and cyst groups (4.9% vs. 6.5%, p = 0.243) (Table 1). There was no difference between subjects with a single cyst and those with multiple cysts (p = 0.617). The prevalence of albuminuria was higher in the cyst group than in the control group (11.3% vs. 4.5%, p < 0.001) (Table 1). However, there was no difference in the prevalence of albuminuria between the single and multiple cyst groups (12.0% vs. 7.1%, p = 0.363). Additionally, we divided the subjects with renal cysts into four subgroups according to cyst location (cortex, medulla, parapelvic region, and multiple regions). There was no significant difference in the prevalence of albuminuria among the four subgroups (p = 0.813) (Fig. 1).
To evaluate the effect of simple cysts on albuminuria, we conducted logistic regression analyses of albuminuria. Univariate analyses demonstrated that male sex, BMI, abdominal girth, systolic BP, diastolic BP, hypertension, diabetes mellitus, HbA1c, eGFR, presence of a renal cyst, serum triglyceride level, HDL-C level, LDL-C level, and uric acid level were significantly associated with the presence of albuminuria (Table 3). To verify that simple renal cysts are independently associated with albuminuria, multivariate regression analysis was performed, which revealed that the presence of simple renal cysts was associated with a 2.307-fold increased risk of albuminuria, independent of other risk factors (95% CI, 1.512 to 3.519; p < 0.001).
We performed interaction analyses between simple cysts and other variables (such as age, male sex, systolic BP, history of hypertension, serum triglyceride level, and uric acid level) and found no significant interaction.
After 1:2 propensity score matching, 275 pairs were generated (275 in cyst group and 550 in control group) and the standardized mean differences were less than 0.1 for most variables (except serum triglyceride level). There were no differences in the baseline characteristics of the matched groups (Supplementary Table 1). The prevalence of albuminuria was higher in the cyst group than in the control group (10.9% vs. 6.2%, p = 0.017). Logistic regression analyses of albuminuria in matched cohorts confirmed that the presence of simple renal cysts was associated with increased odds of albuminuria (OR, 1.858; 95% CI, 1.112 to 3.107; p = 0.0182) (Supplementary Table 2).
Our study demonstrated that age and high BP were independent risk factors for simple renal cysts in adults younger than 40 years, similar to older populations. With respect to the relationship between cysts and renal parenchymal injury, the presence of renal cysts was not associated with an increased prevalence of hematuria. This finding suggested that renal cysts have a very low probability of causing hematuria. Conversely, subjects with simple renal cysts were more likely to have albuminuria, independent of the classic risk factors. The causal relationship between simple renal cysts and albuminuria needs to be elucidated in further studies.
There is a growing body of evidence showing that simple renal cysts are closely related to hypertension. Several mechanisms linking hypertension and renal cysts have been suggested [6ŌĆō11,14,15]. Lee et al. [9] reported that serum renin levels are increased in subjects with large or multiple cysts, suggesting that the renin-angiotensin-aldosterone system may be a mediator of high BP in individuals with renal cysts. On the other hand, both hypertension and renal cysts may be manifestations of the loss of functional nephrons due to aging or progressive renal diseases. Hollenberg [16] found that patients with hypertension have fewer glomeruli and larger glomerular volume than the matched normotensive controls, supporting the hypothesis that a decreased number of nephrons may lead to the development of primary hypertension. Simultaneously, compensatory tubular cell hypertrophy and hyperplasia may occur in the remaining nephrons, causing cyst formation [5,17].
Albuminuria is considered a marker of kidney disease that is a manifestation of early glomerular injury or endothelial dysfunction [18,19]. We found that the prevalence of albuminuria increased in patients with simple renal cysts. This cross-sectional association suggested that simple renal cysts might induce early renal parenchymal injury in the young population. According to previous studies, cortical and medullary cysts may obstruct nephrons and reduce renal function in patients with ADPKD [20]. Further, studies using an animal model of ADPKD have indicated that albuminuria results from the defect in endocytosis and the lack of reabsorption of low-molecular-weight proteins in the cyst-lining epithelium of the proximal tubules [21]. Another study that involved biochemical analysis of cyst fluid suggested that most simple renal cysts originate from glomerular proximal tubules [22]. Additionally, copeptin, a marker of urinary albumin excretion and disease severity of ADPKD, has been associated with an increased prevalence of simple renal cysts in the general population [23]. A large prospective cohort study suggested that plasma copeptin levels are associated with urinary albumin excretion [24]. Thus, the mechanism of the occurrence of albuminuria in patients with simple renal cysts might be similar to that of ADPKD.
A few studies have examined the relationship between simple renal cysts and renal dysfunction. Chang et al. [3] have shown that renal cysts are associated with elevated serum creatinine levels. In another study, the presence of renal cysts was clinically associated with lower eGFR in hospitalized patients aged < 60 years [13]. In our study, eGFR was not associated with the presence of renal cysts. The discrepancy between our findings and those of previous studies can be attributed to the fact that our study subjects were participants of health examinations, not patients diagnosed with chronic kidney disease (CKD). Almost all subjects in this study had normal kidney function and the median eGFR was > 100 mL/min/1.73 m2 in both groups. In patients with CKD, renal cysts are more frequently observed with CKD progression [5].
Our study has several limitations. First, this was a cross-sectional study that only proved an association, rather than a causal relationship, between albuminuria and simple renal cysts. Therefore, we could not ascertain the long-term outcomes of albuminuria in association with simple renal cysts. Further, we hypothesized that albuminuria in patients with simple cysts has a similar mechanism of that of albuminuria in patients with ADPKD; however, there was no significant difference in the prevalence of albuminuria according to the number of simple cysts. Further studies using a larger number of patients with single and multiple simple cysts are required to investigate our hypothesis. Second, we included adults younger than 40 years to minimize the effects of aging and to prove the significance of simple renal cysts in the young population. However, most of the study subjects were in their late 30s. As the number of young subjects who underwent health examinations was small, it was not possible to determine the clinical significance of simple renal cysts in individuals in their 20s. Third, owing to the retrospective nature of this study, there might be some missing data. We could not obtain information on other potential confounding factorsŌĆösuch as family history of kidney diseases or hypertension.
In conclusion, age and hypertension were independent predictors of simple renal cysts in adults younger than 40 years. Renal cysts were not associated with an increased prevalence of hematuria. However, the presence of renal cysts was associated with a 2.30-fold increased risk of albuminuria. The causal relationship between simple renal cysts and albuminuria needs to be elucidated in further studies.
Figure┬Ā1
Prevalence of albuminuria according to cyst location. There is no difference in the prevalence of albuminuria according to the cyst location (cortex, medulla, parapelvic region, and multiple; p = 0.813).
Table┬Ā1
Baseline characteristics of the subjects according to simple cyst status
Table┬Ā2
Predictors of the presence of simple cysts in univariate and multivariate logistic regression analyses
Table┬Ā3
Univariate and multivariate logistic regression analyses of albuminuria
REFERENCES
2. Terada N, Arai Y, Kinukawa N, Terai A. The 10-year natural history of simple renal cysts. Urology 2008;71:7ŌĆō11.


3. Chang CC, Kuo JY, Chan WL, Chen KK, Chang LS. Prevalence and clinical characteristics of simple renal cyst. J Chin Med Assoc 2007;70:486ŌĆō491.


4. Terada N, Ichioka K, Matsuta Y, Okubo K, Yoshimura K, Arai Y. The natural history of simple renal cysts. J Urol 2002;167:21ŌĆō23.


6. Chin HJ, Ro H, Lee HJ, Na KY, Chae DW. The clinical significances of simple renal cyst: is it related to hypertension or renal dysfunction? Kidney Int 2006;70:1468ŌĆō1473.


7. Hong S, Lim JH, Jeong IG, Choe J, Kim CS, Hong JH. What association exists between hypertension and simple renal cyst in a screened population? J Hum Hypertens 2013;27:539ŌĆō544.


8. Kim SM, Chung TH, Oh MS, Kwon SG, Bae SJ. Relationship of simple renal cyst to hypertension. Korean J Fam Med 2014;35:237ŌĆō242.



9. Lee CT, Yang YC, Wu JS, et al. Multiple and large simple renal cysts are associated with prehypertension and hypertension. Kidney Int 2013;83:924ŌĆō930.


10. Luscher TF, Wanner C, Siegenthaler W, Vetter W. Simple renal cyst and hypertension: cause or coincidence? Clin Nephrol 1986;26:91ŌĆō95.

11. Pedersen JF, Emamian SA, Nielsen MB. Significant association between simple renal cysts and arterial blood pressure. Br J Urol 1997;79:688ŌĆō691.


12. Lee HS, Kim SG, Kim EJ, et al. The changes of renal function in apparent healthy individuals with a simple renal cyst. Kidney Res Clin Pract 2007;26:554ŌĆō558.
13. Al-Said J, Brumback MA, Moghazi S, Baumgarten DA, OŌĆÖNeill WC. Reduced renal function in patients with simple renal cysts. Kidney Int 2004;65:2303ŌĆō2308.


14. Choi JD. Clinical characteristics and long-term observation of simple renal cysts in a healthy Korean population. Int Urol Nephrol 2016;48:319ŌĆō324.


15. Pedersen JF, Emamian SA, Nielsen MB. Simple renal cyst: relations to age and arterial blood pressure. Br J Radiol 1993;66:581ŌĆō584.


16. Hollenberg NK. Nephron number in patients with primary hypertension. Curr Hypertens Rep 2003;5:285ŌĆō286.

17. Simms RJ, Ong AC. How simple are ŌĆśsimple renal cystsŌĆÖ? Nephrol Dial Transplant 2014;29(Suppl 4):iv106ŌĆōiv112.



18. Gansevoort RT, Matsushita K, van der Velde M, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 2011;80:93ŌĆō104.



19. Glassock RJ. Is the presence of microalbuminuria a relevant marker of kidney disease? Curr Hypertens Rep 2010;12:364ŌĆō368.



20. Galarreta CI, Grantham JJ, Forbes MS, Maser RL, Wallace DP, Chevalier RL. Tubular obstruction leads to progressive proximal tubular injury and atubular glomeruli in polycystic kidney disease. Am J Pathol 2014;184:1957ŌĆō1966.



21. Obermuller N, Kranzlin B, Blum WF, Gretz N, Witzgall R. An endocytosis defect as a possible cause of proteinuria in polycystic kidney disease. Am J Physiol Renal Physiol 2001;280:F244ŌĆōF253.


22. Scarpa RM, Sorgia M, De Lisa A, et al. Simple renal cysts, biochemical analysis of the cystic fluid, and comparison with blood parameters. Arch Ital Urol Nefrol Androl 1991;63:113ŌĆō117.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print



