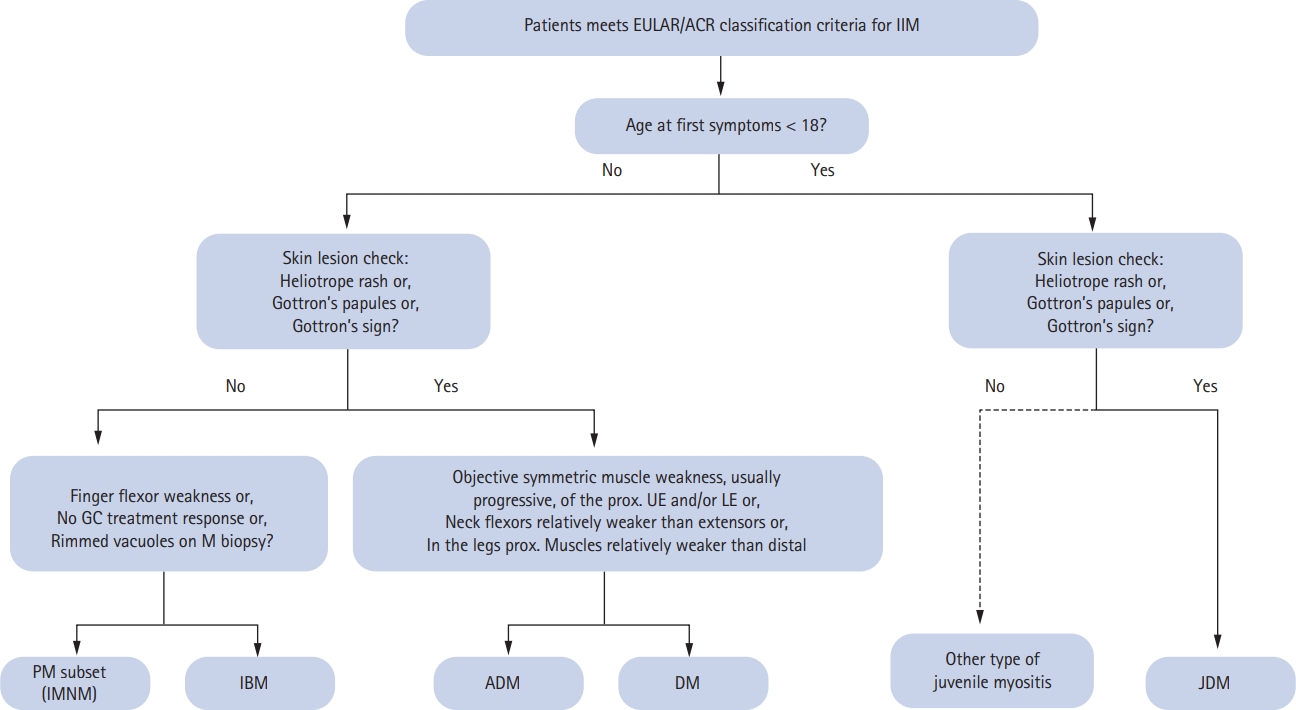
Patients with idiopathic inflammatory myopathies (IIM), including dermatomyositis (DM), polymyositis (PM), inclusion body myositis (IBM), and juvenile idiopathic myositis, show heterogeneous clinical symptoms that reflect differences in organ involvement, but all of these conditions include muscle weakness [1]. Among rheumatic diseases, the diagnosis of IIM can be complicated and both clinicians and investigators have struggled to define a homogeneous group of clinical, laboratory and pathologic features that can be used to separate IIM from other conditions, thus facilitating treatment and providing a detailed focus for clinical research. Most classification criteria are based on accumulated clinical and laboratory manifestations that appear with high certainty in patients who have developed or been diagnosed with a specific disease [2-4]. Among major proposed classification criteria for the different forms of IIM, the comprehensive Bohan and Peter [2] criteria for PM and DM have been used extensively in the clinical setting during the last four decades. The criteria distinguish definite from possible PM and DM based on an initial assessment of ŌĆ£characteristic rashesŌĆØ to distinguish DM and then offers more detailed descriptions for five subgroups of PM/DM, including juvenile, overlapping, and cancer-associated forms of myositis. The modification of the Bohan and Peter criteria proposed by Dalakas added criteria for IBM and identified important muscle biopsy features that led to major advances in practice and in clinical trials [3]. Potential markers associated with distinct clinical phenotypes, such as myositis-specific autoantibodies (MSA), can provide important insights but they have not yet been validated for use in the establishment of diagnostic criteria [4]. In 2017, the European League Against Rheumatism/American College of Rheumatology developed classification criteria (2017 EULAR/ACR criteria) for adult and juvenile IIM that were validated in a data-driven analysis of children and adults with different ethnicities who were seen at centers in Europe, America, and Asia [5]. The major subgroups recognized by these criteria were developed through an international multidisciplinary collaboration, the International Myositis Classification Criteria Project (IMCCP) [6]. The 2017 EULAR/ACR criteria employ a scoring system in which a score < 5.3 is used to rule out IIM (non-IIM) [5]. A remarkable difference from previous criteria was the addition of dysphagia and anti-Jo-1 positivity [5,6]. Patients with pathognomonic skin rashes (heliotrope rash, GottronŌĆÖs papules and/or GottronŌĆÖs sign) are considered to have juvenile DM or DM without muscle biopsy findings (Fig. 1) [5]. Nevertheless, the Bohan and Peter criteria are still commonly used to classify IIM due to their reliance on a small number of variables and their convenient application in routine clinical settings [7].
In a recent issue of the Korean Journal of Internal Medicine, Yoo et al. [7] evaluated the concordance rate of the 2017 EULAR/ACR criteria based on a retrospective review of the medical records of 72 PM and 49 DM Korean patients. They first reclassified patients who had been diagnosed with PM and DM according to the Bohan and Peter criteria after a reassessment based on the 2017 EULAR/ACR criteria. The concordance rate of the classification between the two criteria was 95.8% for PM patients and 83.7% for DM patients. Based on the 2017 EULAR/ACR criteria, three of the 72 PM cases were newly reclassified as non-IIM and eight of the 49 DM cases as amyopathic DM. The report then concluded that the 2017 EULAR/ACR criteria were comparable to the Bohan and Peter criteria for classifying PM and DM in Korean patients. Moreover, the authors recommended the preferential application of the older criteria, due to their convenience, but also that muscle biopsy should be considered in a patient without pathognomonic skin rashes and anti-Jo-1 antibodies [7].
Given the rarity and heterogeneity of IIM, the different classification criteria have many limitations. Although the 2017 EULAR/ACR criteria have been updated following data analyses by the IMCCP and then partially validated, the performance of any new classification criteria should be compared with that of previous gold standard criteria based on actual patient characteristics and applications in a real-world setting [8,9]. This was the case in a Japanese study that externally validated the high specificity of the 2017 EULAR/ACR criteria based on comparisons with 420 IIM and 402 non-IIM cases, although the limitations included a low percentage of pediatric patients. The specificity and specificity of the 2017 EULAR/ACR criteria in the Japanese cohort were comparable to those of the Bohan and Peter criteria (88.4% and 88.3%, respectively) [10]. The performance of the 2017 EULAR/ACR criteria compared to the Bohan and Peter criteria was also assessed in a Swedish monocentric cohort of 439 consecutive patients with IIM, with the add-on effect of the variable, interstitial lung disease (ILD), included as well [9]. The authors reported a sensitivity for the 2017 EULAR/ACR criteria of 87.7%. The better performance of those criteria in classifying subgroups of IIM, and a higher sensitivity and specificity, including a high sensitivity for IBM compared with the physicianŌĆÖs diagnosis, were also reported. At our center, by replacing the variable dysphagia with dysphagia or ILD the sensitivity of the 2017 EULAR/ACR criteria for IIM increased by 1.3 percentage points [9].
Although classification criteria are mainly developed for use in clinical research, it is often advantageous to employ diagnostic criteria for clinical use in the evaluation of patients with rheumatic diseases. However, the incorrect use of classification criteria as a diagnostic tool can hinder a diagnosis in some patients. While a caveat to the high concordance between the 2017 EULAR/ACR criteria and the Bohan and Peter criteria (95.8% for PM patients and 83.7% for DM patients) demonstrated by Yoo et al. [7] may be that the sample size was too small to represent IIM Korean patients generally, this finding could be of value in clinical research and in the diagnosis of patients in routine clinical practice in Korea. Currently, the focus of attention is on variables such as MSA antibodies and magnetic resonance imaging findings, which have shown promise in the early diagnosis of IIM and in assessing the treatment response [11]. The development of new classification criteria based on the most up-to-date information, and applicable not only in clinical research but also in clinical practice, is eagerly awaited.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





