Two-year clinical outcomes after discontinuation of long-term golimumab therapy in Korean patients with rheumatoid arthritis
Article information
Abstract
Background/Aims
The aim of this study was to investigate long-term post-discontinuation outcomes in patients with rheumatoid arthritis (RA) who had been treated with tumor necrosis factor-α inhibitors (TNF-αi) which was then discontinued.
Methods
Sixty Korean patients with RA who participated in a 5-year GO-BEFORE and GO-FORWARD extension trials were included in this retrospective study. Golimumab was deliberately discontinued after the extension study (baseline). Patients were then followed by their rheumatologists. We reviewed their medical records for 2 years (max 28 months) following golimumab discontinuation. Patients were divided into a maintained benefit (MB) group and a loss-of-benefit (LB) group based on treatment pattern after golimumab discontinuation. The LB group included patients whose conventional disease-modifying antirheumatic drug(s) were stepped-up or added/switched (SC) and those who restarted biologic therapy (RB).
Results
The mean age of patients at baseline was 56.5 years and 55 (91.7%) were females. At the end of follow-up, 23 (38.3%) patients remained in the MB group. In the LB group, 75.7% and 24.3% were assigned into SC and RB subgroups, respectively. Fifty percent of patients lost MB after 23.3 months. Demographics and clinical variables at baseline were comparable between MB and LB groups except for age, C-reactive protein level, and corticosteroid use. Restarting biologic therapy was associated with swollen joint count (adjusted hazard ratio [HR], 1.90; 95% confidence interval [CI], 1.01 to 3.55) and disease duration (adjusted HR, 1.12; 95% CI, 1.02 to 1.23) at baseline.
Conclusions
Treatment strategies after discontinuing TNF-αi are needed to better maintain disease control and quality of life of patients with RA.
INTRODUCTION
At the dawn of the era of biologic therapy, tumor necrosis factor-α inhibitors (TNF-αi) were the first agents that changed treatment paradigm and guidelines for patients with rheumatoid arthritis (RA) [1]. It has enabled patients with an active disease to achieve rapid and significant clinical improvement, maintain low disease activity (LDA), or enter clinical remission [2]. On the other hand, TNF-αi are well-known to cause adverse events, including bacterial infections, tuberculosis, congestive heart failure, demyelinating syndrome, autoimmune phenomena, and an increased risk of certain malignancies [3]. Increased prescriptions for TNF-αi also have raised medical cost for RA treatment [4].
Therefore, further interest towards developing strategies for tapering or discontinuing biologic therapy have risen, especially for patients who have sustained clinical remission or LDA [5]. Although many studies have investigated approaches to down-titrate or discontinue TNF-αi, it remains difficult to distinguish patients whose TNF-αi can be discontinued and predict who would experience a flare-up or relapse after such modification of their treatment regimen [6–16]. Previously published studies are mostly clinical trials focusing on down-titrating or discontinuing TNF-αi with follow-up periods of a year or less. They might not reflect real-world outcomes.
Among various TNF-αi, golimumab is a human anti-TNF-α monoclonal antibody purified from TNF-α-immunized transgenic mice with altered expression of human IgG [17]. Pivotal trials of golimumab in RA have been conducted in methotrexate (MTX)-naïve patients (GO-BEFORE) and in patients with inadequate responses to MTX (GO-FORWARD) [18,19]. Both trials were conducted on Korean patients followed by a 5-year extension study in which golimumab was discontinued for all participants at the time of study completion.
The aim of the present study was to investigate 2-year outcomes of patients with RA whose golimumab treatment was discontinued after completing the 5-year GO-BEFORE and GO-FORWARD extension study. Possible risk factors leading to subsequent intensive treatment after golimumab discontinuation were also identified.
METHODS
Study population
The ‘Investigation of outcomes after GOLimumab Discontinuation,’ or GOLD, was an observational study involving 10 centers nationwide that analyzed clinical data of Korean RA patients who completed the 5-year GO-BEFORE (NCT00264537) and GO-FORWARD (NCT00264550) extension study [18,19]. The design of the extension study is depicted in Supplementary Fig. 1. Inclusion criteria for the GO-BEFORE and GO-FORWARD study were: patients 18 years or older with active RA; defined as having at least four swollen and four tender joints and at least two of the following four criteria: (1) C-reactive protein (CRP) ≥ 1.5 mg/dL or erythrocyte sedimentation rate (ESR) of ≥ 28 mm/hour; (2) morning stiffness of ≥ 30 minutes; (3) bone erosion by image; and (4) anti-cyclic citrullinated peptide (anti-CCP) antibody positive or rheumatoid factor (RF) positive. To investigate long-term clinical outcomes after golimumab discontinuation, only patients with at least 2 years of follow-up data were included in this study. The protocol for this study was reviewed and approved by the Institutional Review Board of each participating center (H-1408-081-604 for Seoul National University Hospital). Informed consent was obtained from all subjects upon enrollment. This study was conducted in accordance with principles of the Declaration of Helsinki and Good Clinical Practice Guidelines.
Clinical outcomes during a 2-year follow-up after golimumab discontinuation
Treatment regimen after golimumab discontinuation was determined by the attending rheumatologist at their discretion. Use of conventional disease-modifying antirheumatic drug (cDMARD), corticosteroid, and non-steroidal anti-inflammatory drug, and the restart time point/agent of biologic therapy during the follow-up period were analyzed through electronic medical records. For study patients whose 24-month data were unavailable, the observation period was extended up to 28 months.
To identify factors associated with treatment changes after discontinuation of golimumab, patients were divided into three groups based on their 2-year outcomes after discontinuing golimumab. The “maintained benefit” (MB) group was defined as patients who maintained (or tapered) cDMARDs and corticosteroids. The “loss-of-benefit” (LB) group consisted of patients who stepped-up or added/switched cDMARDs (SC group) and those who restarted biologic therapy owing to an active disease (RB group).
Demographic and clinical parameters at the time of golimumab discontinuation
Clinical and laboratory data at the time of golimumab discontinuation (baseline) were collected by reviewing medical records. Data including age, gender, disease duration, swollen joint count (SJC), tender joint count (TJC), patient’s global assessment, physician’s global assessment, pain score, Health Assessment Questionnaire (HAQ) score, RF and anti-CCP antibody titers, CRP level, and ESR level were collected. Disease activity score (DAS) 28-CRP, DAS28-ESR, DAS28 remission rate, and plain X-ray images were obtained.
Statistical analysis
Variables are expressed as median (interquartile range [IQR]), or number (%). Baseline characteristics were analyzed using chi-square test or Fisher’s exact test for categorical variables and Mann-Whitney test for continuous variables. We performed the Shapiro-Wilk test to examine whether the continuous variables were normally distributed. To assess longitudinal trends for treatment pattern from baseline to the 2-year follow-up, linear-by-linear association test was used for categorical variables. Kaplan-Meier analysis was conducted to evaluate the median time to LB and the proportion of patients with sustained clinical benefits at 6-month intervals during the follow-up period. To identify factors associated with restarting biologic therapy after discontinuation of golimumab, Cox proportional hazard regression analyses were conducted, providing unadjusted, adjusted hazard ratios (HRs) and 95% confidence intervals (CIs). The multivariable model included all significant variables with a p value < 0.2 in the univariable model. SPSS version 26.0 (IBM Co., Armonk, NY, USA) was used for all statistical analyses. A p < 0.05 was considered statistically significant.
RESULTS
Clinical features of study patients at the time of golimumab discontinuation (baseline)
A total of 91 Korean RA patients participated in the 5-year extension study GO-BEFORE and GO-FORWARD from July 2006 to March 2012. Of them, 60 patients with 2-year follow-up data were included in the present study (Fig. 1). Characteristics of the study population are presented in Table 1. Median age and disease duration at the time of discontinuation of golimumab or baseline were 52 years (IQR, 43 to 58) and 10.2 years (IQR, 7.5 to 16.3), respectively. Over 90% of patients (91.7%) were females. The DAS28 remission rate was 50.8%. The median patient’s global assessment score was 2.8 (IQR, 1.3 to 5.5) and the physician’s global assessment score was 1.5 (IQR, 0.4 to 2.8). At baseline, 48 (80.0%) patients were on MTX and eight (13.3%) patients were on MTX plus cDMARDs. Thirty-four (56.7%) patients were prescribed with oral corticosteroids (median prednisolone or its equivalent at 5.0 mg/day [IQR, 2.5 to 5.0]).
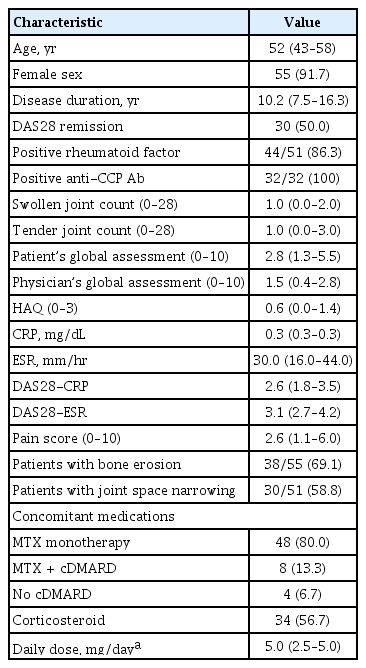
Demographic and clinical characteristics of patients at the time of golimumab discontinuation (n = 60)
Regarding the participated clinical trials, each of the 30 patients from the GO-BEFORE and GO-FORWARD trials was included in this study. Despite the 5-year of extension study, patients from the GO-FORWARD trial had a longer disease duration, higher ESR level and a higher proportion of MTX combination therapy at the time of golimumab discontinuation than those from the GO-BEFORE trial (Supplementary Table 1).
Post-discontinuation clinical outcomes and treatment patterns
Two years after discontinuing golimumab, 23 (38.3%) patients remained in the MB group. Among 37 (61.7%) subjects who fell in the LB group, 28 (75.7%) and nine (24.3%) patients were assigned into SC and RB subgroups, respectively. Biologic agents used in the RB subgroup were adalimumab (n = 4), golimumab (n = 2), sirukumab (n = 2), and infliximab (n = 1).
Treatment patterns at baseline, 1-, and 2-year follow-up periods in MB and LB groups are depicted in Fig. 2. At baseline, the MB group used corticosteroid (73.9% vs. 45.9%, p = 0.03) more frequently than the LB group. The use of hydroxychloroquine tended to increase in the MB group compared to that in the LB group (21.4% vs. 5.4%, p = 0.10). During the 2-year of follow-up period, the use of MTX was increased in the LB group (89.2% at baseline, 94.6% at 1-year follow-up, 100% at 2-year follow-up, p for trend = 0.04). Prescription rates for leflunomide and hydroxychloroquine also gradually increased from baseline to 21.6% (p for trend < 0.01) and 32.4% (p for trend < 0.01), respectively, at 2-year follow-up in the LB group. Additionally, corticosteroid use was increased during the follow-up period (p for trend = 0.03) (Fig. 2). A Kaplan-Meier plot identifying the time point of LB in study patients is shown in Fig. 3. Fifty percent of patients lost MB after 23.3 months (80.0% at 6 months, 61.7% at 12 months, 53.3% at 18 months, and 48.3% at 24 months).

Use of conventional disease-modifying antirheumatic drugs, corticosteroids (CS), and non-steroidal anti-inflammatory drugs (NSAIDs) in the maintained benefit and loss-of-benefit groups during the follow-up period. MTX, methotrexate; LEF, leflunomide; HCQ, hydroxychloroquine; SSLZ, sulfasalazine; TAC, tacrolimus.
Factors related to LB or restarting biologic therapy after golimumab discontinuation
We first compared variables at baseline in MB and LB groups. There was a significant difference in age (p < 0.01) and CRP level (p < 0.01) between the two groups. However, other variables were comparable (Table 2). Therefore, we decided to focus more on identifying factors associated with restarting biologic therapy after discontinuing golimumab. Restarting biologic therapy was associated with disease duration (unadjusted HR, 1.07; 95% CI, 1.01 to 1.13; p = 0.02), SJC (unadjusted HR, 1.26; 95% CI, 1.09 to 1.45; p < 0.01) and TJC (unadjusted HR, 1.18; 95% CI, 1.02 to 1.37; p = 0.02) in univariable analysis (Table 3). However, in multivariable analysis, SJC (adjusted HR, 1.90; 95% CI, 1.01 to 3.55; p = 0.046) and disease duration (adjusted HR, 1.12; 95% CI, 1.02 to 1.23; p = 0.02) at baseline were significant factors associated with resuming biologic therapy.
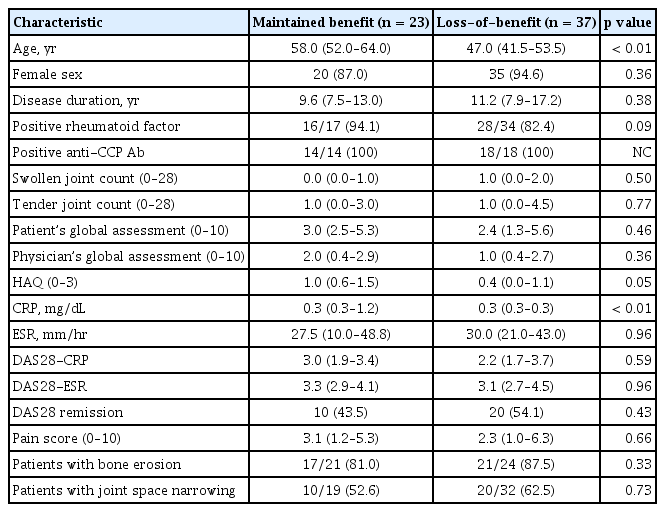
Characteristics of patients with maintained benefit vs. loss-of-benefit at the time of golimumab discontinuation

Cox hazard regression model to identify factors related to restarting biologic therapy after discontinuing golimumab
A subgroup analysis was performed considering the differences in baseline characteristics between patients who participated in the GO-BEFORE and GO-FORWARD trials. Two (6.7%) out of 30 patients in the GO-BEFORE group and seven (23.3%) out of 30 patients in the GO-FORWARD group resumed biologic therapy during follow-up after discontinuation of golimumab. However, due to the small number of patients in each group, there were no significant factors associated with restarting biologic therapy in the subgroup analysis (data not shown).
DISCUSSION
Even with recent advances in treatment recommendations and strategies for RA, discontinuing or tapering TNF-αi is a challenging decision to make. A consensus is needed among rheumatologists as to when this should be done. Our study aimed to analyze clinical outcomes in patients who discontinued golimumab after a 5-year extension open-label trial. In our study, 38.3% of patients who achieved LDA or remission (43.5% of study subjects were in DAS28 remission) maintained or tapered cDMARDs during 2 years after discontinuing golimumab without stepping-up cDMARD use or restarting biologic therapy. To the best of our knowledge, this is the first report to present data of a 2-year MB in RA patients after discontinuing a single biologic agent used for 5 years.
Outcomes after biologic discontinuation shown in previous clinical studies are relatively heterogeneous. Tanaka et al. [9] reported that 55% of patients with RA who discontinued infliximab after attaining LDA (DAS28 < 3.2) maintained stable disease activity at a 1-year follow-up while 45% of patients experienced disease flare-up. A study investigating patients who achieved DAS28 remission after using adalimumab showed that 48% of subjects were able to maintain DAS28 remission for 1 year after discontinuing adalimumab [7]. Of note, Saleem et al. [20] reported that only 15% of patients who underwent delayed (median symptom duration of 9 months). TNF-αi treatment were able to attain sustained DAS28 remission after its discontinuation and that a shorter duration of untreated symptoms was the main predictor of successful cessation. Harigai et al. [21] demonstrated that 18.2% of long-standing RA patients who discontinued adalimumab after attaining LDA maintained similar disease activity for 1 year without resuming biologic therapy. Furthermore, Brocq et al. [22] reported that, among patients with long-standing RA who had achieved DAS28 remission for at least 6 months, 75% experienced disease relapse within 12 months after discontinuing TNF-αi. Results of previous studies utilizing clinical disease activity index (CDAI) were dissimilar. Smolen et al. [13] showed that only 17.6% of established RA patients who stopped certolizumab pegol maintained CDAI remission until week 52 and suggested that it would be difficult for patients to withdraw from certolizumab pegol even if they had achieved remission. According to data from the Corrona registry, 42% of RA patients who exhibited disease activity of LDA or lower (CDAI≤10) successfully discontinued their first TNF-αi and experienced an MB for 24 months [6]. Despite the fact that baseline disease activity and cDMARD/corticosteroid use in these studies were different from those in our study, results of the Corrona data are similar to what we demonstrated.
In the multivariable logistic regression analysis, SJC and disease duration at the time of golimumab discontinuation were significant factors associated with restarting biologic therapy after golimumab discontinuation. For every 1-point increase in SJC, the chance of resuming biologics increased 8.34-fold. Previous studies have shown that higher level of disease activity at the time of tapering biologics and presence of RF or anti-citrullinated peptide antidbody may reduce the success of dose-reduction regimen [16,23]. In a study of Tanaka et al. [24], patients achieving sustained deep or stringent remission with biologics had a higher probability of remaining in remission or LDA after dose reduction or withdrawal of etanercept. Interestingly, corticosteroids were used more frequently in the MB group than in the LB group at the time of golimumab discontinuation, though the mean dose of prednisolone was below 5 mg/day. This suggests that concomitant use of low-dose corticosteroids may have aided in maintaining LDA or a stable disease state and a longer biologic-free period, although European Alliance of Associations for Rheumatology (EULAR) guidelines recommend to start with tapering steroids, then biologics, followed by cDMARDs [25].
Given that two-thirds of patients lose their good disease state after discontinuation of golimumab in this study, gradual tapering biological disease-modifying antirheumatic drug rather than discontinuation may be needed to maintain a long-term clinical response in patients with RA in remission or LDA. On the other hand, recent studies showed non-inferiority of tapering or stopping cDMARD versus maintaining cDMARD therapy in stable patients who treated with cDMARD plus biologic therapy [26,27]. In a study comparing cDMARD tapering and TNF-αi tapering, cDMARD tapering tended to be more effective by a 10% difference in flare rate [28], underscoring the need for further research to assess the DMARD tapering strategy in patients with RA.
Our study was the first to determine patient outcomes after golimumab discontinuation for a relatively long period of time (2 years). In addition, our patients had been treated with a single TNF-αi at regular intervals for an extensive period. However, our study has several limitations. First, the study population was relatively small. Of the 91 patients who participated in the GO-BEFORE and GO-FORWARD trials, 31 were excluded from this study due to lack of 2-year follow-up data. Therefore, the MB group may have been underestimated in evaluating long term clinical outcomes after discontinuation of golimumab. In addition, the list of medications and disease activity at baseline were heterogeneous. Furthermore, most patients showed mild to moderate disease activity at the time of golimumab discontinuation. Most previous studies investigated patients who had experienced LDA or remission for at least 6 months prior to TNF-αi discontinuation. However, our higher baseline disease activity was not associated with an increased incidence of restarting biologic therapy compared to other studies. This in part might be due to the national health insurance reimbursement policy that requires a period of cDMARD combination therapy before restarting biologic therapy. Second, as this was a retrospective analysis, there were some missing data on disease activity during the 2-year follow-up period. Therefore, we used indirect measures of disease flare-up or relapse by assessing stepped-up/added-on/switched cDMARDs or restarting biologic therapy which reflected uncontrolled disease activity. Third, the baseline parameter was the time point of discontinuation of golimumab. Initial and earlier responses to golimumab (i.e., American College of Rheumatology response at 52 weeks, duration of remission, and timing of clinical response) were not considered. However, these indices were too remote to directly affect clinical outcomes after discontinuation.
In summary, controlling disease activity of patients with RA is a long-term, unremitting process that is in part affected by underlying clinical characteristics at key time points of treatment. Beyond guidelines on when to start TNF-αi, post-discontinuation treatment strategies are needed to better maintain disease control and quality of life of patients with RA.
KEY MESSAGE
1. During a 2-year period after golimumab discontinuation, one-third of patients with rheumatoid arthritis were able to maintain or taper conventional disease-modifying antirheumatic drugs, whereas 15% of patients resumed biologic therapy.
2. Patients with higher swollen joint count or greater disease duration at the time of golimumab discontinuation were apt to resume biologic therapy.
3. Treatment guidelines aiming at sustained disease activity are needed for patients who discontinue long-term biologic therapy.
Acknowledgments
This study was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), Ministry of Health & Welfare, Republic of Korea (grant number: HI14C1277), Korea Healthcare Technology R&D Project, Ministry of Healthcare & Welfare, Republic of Korea (HI10C2020), and the Ministry of Science, ICT and Future Planning, Republic of Korea (NRF-2015M3A9B6052011, NRF-2020M3E5E2037430).
Notes
No potential conflict of interest relevant to this article was reported.



