 |
 |
| Korean J Intern Med > Volume 37(3); 2022 > Article |
|
See editorial "Is radiological healing alone enough? ‘Can’t take my eyes off’ the mucosa" on page 551.
Abstract
Background/Aims
The optimal tools for monitoring Crohn’s disease (CD) are controversial. We compared radiology plus ileocolonoscopy and radiology alone in terms of prognosis prediction and evaluated the agreement between radiologic and ileocolonoscopic findings in patients with CD.
Methods
Patients with CD who were followed up with computed tomography enterography (CTE) or magnetic resonance enterography (MRE) alone or CTE or MRE plus ileocolonoscopy were retrospectively recruited. Time to relapse was investigated to evaluate the difference in prognosis using the log-rank and Cox regression tests, and the agreement between radiologic and ileocolonoscopic findings was determined using a kappa value.
Results
A total of 501 patients with CD in clinical remission who underwent CTE or MRE and/or ileocolonoscopy were analyzed. Of these, 372 (74.3%) patients underwent CTE or MRE alone and 129 (25.7%) patients underwent CTE or MRE plus ileocolonoscopy. The cumulative maintenance rate of clinical remission between the two groups was not significantly different (p = 0.526, log-rank test). In multivariate analysis, age <40 years (hazard ratio [HR], 2.756; 95% confidence interval [CI], 1.263 to 6.013) and a history of steroid use (HR, 2.212; 95% CI, 1.258 to 3.577) were found to independently predict an increased risk for clinical relapse in patients with CD in clinical remission. Radiologic and ileocolonoscopic findings had a moderate degree of agreement (κ = 0.401, −0.094 to 0.142). The comparison of agreement between radiologic and ileocolonoscopic findings was the highest in the anastomotic site (κ = 0.749, −0.168 to 0.377).
Crohn’s disease (CD) is a chronic progressive immune-mediated intestinal disease characterized by transmural inflammation [1]. As CD progresses over a long period, it requires proper treatment targets and continuous monitoring because of repeated recurrence and remission, eventually leading to structural bowel damage. Since biologics have emerged as a key treatment option for CD, mucosal healing has become the most important treatment target [2]. Deep remission, which combines endoscopic and clinical indicators of disease activity, is an important therapeutic goal in CD. It is defined as Crohn’s Disease Activity Index (CDAI) < 150 and complete mucosal healing without mucosal ulcerations [3]. Moreover, transmural inflammatory healing has been associated with lesser inflammatory activity, lesser escalation of therapy, lesser corticosteroid use, a lower of hospitalization, and a reduced need for surgery than endoscopic healing alone. Achieving deep remission with transmural inflammatory healing requires repeated endoscopic and radiologic approaches.
Ileocolonoscopy has traditionally been considered the standard tool for the diagnosis and management of CD as it is useful in evaluating the disease extent and activity, determining the therapeutic response, performing surveillance for dysplasia, and determining the therapeutic procedure. However, there is a poor correlation between symptom scores and the degree of endoscopic inflammation as well as between clinical remission and mucosal healing [4]. In the case of intestinal obstruction or stricture, the examination is limited because endoscopic access is challenging [5,6]. Repeated ileocolonoscopy, which is a relatively invasive procedure, has low patient acceptability, and there is a potential risk of endoscopy-associated perforation [7].
Currently, several cross-sectional imaging modalities, such as computed tomography (CT) and magnetic resonance imaging, have widely replaced traditional modalities [8,9]. Computed tomography enterography (CTE) and magnetic resonance enterography (MRE) have several advantages in terms of monitoring patients with CD, such as better acceptability than ileocolonoscopy, transmural assessment of both the small bowel and colon, and detection of extra-enteric complications, including bowel obstructions and distensions, abscesses, fistulas, and mesenteric and perienteric fat [9]. Preliminary data have also suggested that MRE could be a reliable tool to monitor therapeutic efficacy [10,11]. MRE performed with no enteroclysis, no bowel cleansing the day before the examination, and no rectal enema demonstrated high accuracy in CD and is preferred to CT because of its lack of ionizing radiations [12,13]. However, a large volume of enteric contrast medium is required to achieve adequate luminal distension during CTE or MRE. Owing to the long acquisition time associated with MRE, antiperistaltic medications such as intravenous butyl scopolamine should be used. Non-invasive quantification of fibrosis is much more challenging, as cross-sectional imaging cannot accurately quantify the fibrotic component of an otherwise easy-to-diagnose stricture. In particular, to perform both ileocolonoscopy and radiologic tests, it is challenging to perform them on the same day, and there is a hassle of having to take a bowel preparation twice. It is also more expensive and takes longer, making it questionable whether it is necessary to perform both tests for monitoring patients with CD.
To date, the methods to manage CD are both to improve the quality of life and appropriately treat and predict the prognosis using proper monitoring tools. However, no data have been reported regarding this issue, and the most efficient methods are still controversial. We need an optimized tool for monitoring individual patients with CD. Therefore, this study primarily aimed to find an appropriate method as a monitoring tool to confirm whether performing radiology plus ileocolonoscopy is necessary and whether it predicts prognosis better than radiology alone. Additionally, we attempted to determine the degree of agreement between the two tests.
This single-center retrospective study evaluated patients with CD in clinical remission who underwent CTE or MRE or ileocolonoscopy at Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea between November 2005 and December 2019. The diagnosis of CD was confirmed based on clinical presentations and a combination of endoscopic, radiologic, and histological criteria [14].
The inclusion criteria were as follows: (1) patients aged ≥ 18 years; (2) eligible patients who underwent ileocolonoscopy within 3 months of CTE or MRE or vise versa; and (3) outpatient care within 3 months of CTE or MRE. The exclusion criteria were as follows: (1) patients aged < 18 years; (2) inaccurate or inconclusive diagnosis of CD; (3) > 3-month interval between CTE or MRE and ileocolonoscopy; and (4) > 3-month interval between CTE or MRE and follow-up. Medical records of the patients were retrospectively reviewed.
Patients were advised to follow a soft or clear liquid dietary regimen 24 to 72 hours before colonoscopy according to their bowel habits. Ileocolonoscopy was performed after standard bowel preparation using 2 L of polyethylene glycol electrolyte solution (Coolprep, Taejoon Pharmaceuticals, Seoul, Korea). During endoscopy, patients received a standard dose of the sedative; 5 mg of midazolam or 40 mg of propofol. Ileocolonoscopy was performed by a gastroenterologist with a conventional colonoscope (CF H260AL or CF H260AI, Olympus Optical, Tokyo, Japan). The performing endoscopist was aware of the patient’s history, and the colonoscope was introduced in the terminal ileum except that the scope could not pass due to stenosis. Endoscopic remission was defined as the simple endoscopic score for CD (SES-CD) of less than 4 [15].
CTE was performed after 4 hours of fasting and oral administration of 1,250 mL of a polyethylene glycol solution (Coolprep). CTE was performed using a multi-detector CT (SOMATOM Definition Flash, Siemens Healthineears, Erlangen, Germany). The parameters were set as follows: 240 mA, 100 kVp, 0.5 second tube rotation time, and 0.625 pitch. The intravenous contrast material (0.2 mL/kg, maximum of 150 mL) iohexol 300 mgI/mL (Hexosure, Pharvis Korea, Seoul, Korea) was administered at a rate of 3 mL/sec. For routine CTE in patients without gastrointestinal bleeding, a single portal venous phase (typically 60 seconds) was obtained. For patients with suspected gastrointestinal bleeding, multiphase CT consisted of unenhanced, arterial, and portal venous phases. A bolus-tracking method was used to commence the diagnostic CT data acquisition after intravenous injection of the contrast agent. CTE images were reconstructed in axial and coronal planes with 3-mm thickness and no interslice gap.
MRE was performed after 6 hours of fasting and oral administration of 1,250 mL of a sorbitol solution for luminal distension of the bowel before scanning. All patients were examined in a supine position using a 3-T system (Ingenia CX or Ingenia Elition X, Philips Healthcare, Best, the Netherlands). MRE sequences consisted of coronal T2-weighted half-Fourier sequences with and without fat suppression, axial T2-weighted half-Fourier sequences with fat suppression, coronal diffusion-weighted imaging (with b factors of 0 and 800 sec/mm2) and an apparent diffusion coefficient (ADC) map, and coronal T1-weighted spoiled gradient-echo sequences with fat suppression, including unenhanced imaging and dynamic phases sequences performed after intravenous administration of gadolinium-based contrast material (Gadovist, Schering, Berlin, Germany) (0.2 mmol/kg at a rate of 2 mL/sec), followed by a saline bolus injection, and an axial delayed contrast-enhanced T1-weighted spoiled gradient-echo sequence with fat suppression (typically 28 seconds with breath-hold). A dose of 10 mg scopolamine-N-butyl bromide (Buscopan, Boehringer Ingelheim, Ingelheim, Germany) was administered intravenously twice at intervals during the scan to reduce bowel peristalsis. The examination was performed without bowel cleansing or rectal enema. CTE or MRE findings were prospectively interpreted by one of six experienced abdominal radiologists.
MRE findings indicating active inflammation were defined as follows: segmental mural hyperenhancement (increased mural signal intensity at MRE on contrast material), intramural edema (identified when hyperintense signal in the bowel wall is present on fat-suppressed T2-weighted images), and/or wall thickening (subdivided as mild 3 to 5 mm, moderate > 5 to 9 mm, or severe ≥ 10 mm). Mesenteric findings (perienteric edema and/or inflammation, engorged vasa recta, fibrofatty proliferation, mesenteric venous thrombosis and/or occlusion, lymphadenopathy) or penetration (sinus tract, fistula, inflammatory mass, abscess, or free perforation) [16,17]. CTE findings indicating active inflammation were defined as follows: segmental mural hyperenhancement (increased mural attenuation at CTE on contrast material), intramural edema (enhanced outer serosal and inner mucosal layers and an interposed submucosal layer of lower attenuation), and/or bowel wall thickening (> 3 mm), comb sign (prominence of the vasa recta adjacent to the inflamed loop of bowel), bowel stricture, or penetration [18]. Chronic disease changes without active inflammatory findings were considered remission. CTE or MRE remission, defined as lack of active inflammation, was considered as complete CTE or MRE healing [19].
A retrospective review of medical records was performed to comprehensively obtain the updated data on the baseline and follow-up patient characteristics, laboratory findings, clinical disease activity as assessed by the CDAI, current medications, complications, etc. Patients receiving mesalazine or sulfasalazine were considered to be on 5-aminosalicylic acids (5-ASAs). Patients receiving azathioprine, mercaptopurine, cyclosporine, or methotrexate were considered to be on immunomodulatory therapy, whereas those receiving infliximab, adalimumab, golimumab, vedolizumab, or ustekinumab were considered to be on biologic monotherapy. The combination therapy was considered to be a combination of immunomodulatory therapy and biologic therapy. The CDAI was used to determine clinical disease activity in patients before CTE or MRE and ileocolonoscopy at the time of outpatient visit. The CDAI is an assessment tool based on routine questions by a gastroenterologist during the patient visit to measure disease activity. The CDAI is a numerical calculation derived from the sum of products comprising a list of eight items, including the number of liquid stools, abdominal pain, general well-being, extraintestinal complications, antidiarrheal drugs, abdominal mass, hematocrit, and body weight. The patients were grouped according to the clinical activity of disease: remission (CDAI < 150), mild activity (CDAI 150 to 219), moderate activity (CDAI 220 to 449), and severe (CDAI ≥ 450) [20]. Clinical relapse was defined as worsening of CD-related symptoms (increase in CDAI more than 150), hospitalization, the need for corticosteroids or surgery [21].
The study protocol was consistent with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board of Yonsei University Health System (IRB No. 4-2021-0372). Informed consent was not required due to the retrospective study design.
Continuous variables are reported using descriptive statistics as the median and interquartile range (IQR). Comparisons were performed using Student’s t tests for continuous variables and the chi-square test for categorical variables. The clinical relapse-free survival was calculated using the Kaplan-Meier method. Univariate and multivariate logistic regression analyses were performed to identify factors predictive of clinical relapse of CD using log-rank tests and Cox models, respectively. The results are expressed as hazard ratios (HRs) with 95% confidence intervals (CIs). The concordance between CTE or MRE and ileocolonoscopy were studied using kappa statistics and proportion of accuracy (%) for categorical parameters. Cohen’s kappa (κ) was calculated to assess the agreement in the case of categorical variables. Cohen’s kappa coefficient indicates reproducibility of interpretations; κ > 0.80 is considered excellent, 0.80 ≥ κ > 0.60 is considered good, 0.60 ≥ κ > 0.40 is considered moderate, 0.40 ≥ κ ≥ 0.00 is considered average, and κ < 0.00 is considered poor [22]. The kappa coefficients were recorded with their CIs. Statistical analyses were performed using IBM SPSS Statistics for Windows version 25.0 (IBM Corp., Armonk, NY, USA). A p < 0.05 indicated statistical significance.
Of a total of 952 patients who underwent CTE or MRE and/or ileocolonoscopy after the diagnosis of CD, 501 patients in clinical remission were enrolled and analyzed (Fig. 1). Of them, 372 (74.3%) patients underwent CTE or MRE alone and 129 (25.7%) patients underwent CTE or MRE plus ileocolonoscopy. The baseline characteristics of the study population are summarized in Table 1. The median patient age was 29.8 years, and the median interval from diagnosis to CTE or MRE was 23.9 months (IQR, 1.1 to 71.1). According to the Montreal classification, 253 (50.5%) patients had ileocolonic involvement (L3), 355 (70.9%) had non-stricturing, non-penetrating behavior, and 177 (35.3%) had evidence of perianal disease. Fifty-five (11.0%) patients were current smokers, while 74 (14.8%) were former smokers. Previous intestinal resection surgery was performed in 155 (30.9%) patients, and 52 (10.4%) patients were receiving systemic steroids. Further, 230 (45.9%) patients were treated with immunomodulators, and 30 (6.0%) patients were receiving biologic monotherapy. The median erythrocyte sedimentation rate, serum C-reactive protein level, and serum albumin level were 34.0 mm/hr, 11.5 mg/dL, and 4.0 g/dL, respectively.
Univariate analyses showed that age < 40 years and the use of steroids were significantly associated with clinical relapse of CD (all p < 0.05) (Table 2). In addition, there was a marginally significant relationship in terms of clinical relapse when the presence of active inflammation in one of the two tests, and active inflammation in both tests was compared to remission in CTE or MRE plus ileocolonoscopy (p = 0.056 and p = 0.050 by log-rank test, respectively) (Fig. 2). However, the cumulative incidence rate of clinical relapse in the radiology only group was not significantly higher than that of the radiology and ileocolonoscopy group (p = 0.526, log-rank test) (Fig. 3). Subsequent multivariate analysis adjusted for the CTE or MRE showed that age < 40 years (HR, 2.756; 95% CI, 1.263 to 6.013) and the use of steroids as an ongoing medication (HR, 2.121; 95% CI, 1.258 to 3.577) were independently associated with predicting increased risk for clinical relapse in patients with CD in clinical remission (all p < 0.05); however, the performance of CTE or MRE plus ileocolonoscopy were not compared with CTE or MRE alone (HR, 1.316; 95% CI, 0.799 to 2.168).
In our study, CTE or MRE found active inflammation in 171 (81.4%) patients, and ileocolonoscopy found active inflammation in 126 (60.0%) patients. Radiology and ileocolonoscopy had a moderate degree of agreement (κ = 0.401, −0.094 to 0.142) (Table 3). We analyzed the characteristics of the patients with discrepant results between radiology and ileocolonoscopy for active inflammation in Table 3 (Supplementary Table 1). In addition, in the 121 cases of active inflammation observed with both radiology and ileocolonoscopy, the colon was divided into eight segments, including the anastomotic site, and the degree of agreement was compared. There were 66 (44.9%) terminal ileal, seven (4.8%) cecal, 14 (9.5%) ascending colon, 11 (7.5%) transverse colon, nine (6.1%) descending colon, 10 (6.8%) sigmoid colon, and four (2.7%) rectal involvements in the segmental evaluation by CTE or MRE. There were 58 (24.1%) terminal ileum, 14 (5.8%) cecal, 26 (10.8%) ascending colon, 21 (8.7%) transverse colon, 20 (8.3%) descending colon, 29 (12.0%) sigmoid colon, and 24 (10.0%) rectal involvements in the segmental evaluation by ileocolonoscopy. Radiology and ileocolonoscopy had a moderate degree of agreement in the terminal ileum and cecum (κ = 0.412, −0.173 to 0.266; and κ = 0.409, −0.219 to 0.334). The agreement between radiologic and ileocolonoscopic findings was higher in the anastomotic site (κ = 0.749, −0.168 to 0.377) than in other ileocolonic segments (Table 4).
Accurate assessment of the extent and activity of CD remains a problem and often requires a combination of clinical, biochemical, endoscopic, histologic, and conventional radiologic techniques. A treat-to-target strategy and rigorous monitoring of inflammation have recently been recommended to manage patients with CD [23–25]. Although in the past, ileocolonoscopy was generally considered the most accepted gold standard for evaluating inflammatory lesions in patients with CD [26], cross-sectional radiology provides new and complementary information compared with mucosal assessment by optical ileocolonoscopy [27,28]. In particular, it is not yet clear whether additionally performing ileocolonoscopy is advisable when monitoring with radiology. Therefore, this study focused on how well the prognosis can be predicted by dividing the cases with radiology alone and those with both radiology and ileocolonoscopy [29]. If there is no significant difference in clinical relapse when CTE or MRE and ileocolonoscopy are performed, and only CTE or MRE is performed, there is no need to perform the two tests at the same time. Furthermore, we analyzed the degree of correspondence between radiology and ileocolonoscopy for each segment of the bowel. If there is a high degree of agreement between the two test methods, only one of the two tests can be performed and monitored.
We found that the presence of active inflammation on radiology or ileocolonoscopy was significantly associated with poor prognosis. However, both radiology and ileocolonoscopy were not significantly different from radiology alone in predicting the prognosis of CD. Moreover, age, younger than 40 years and the use of steroids were associated with a poor prognosis of CD. Lopes et al. [30] showed that CTE findings and fecal calprotectin (FC) significantly correlated with endoscopic findings. These two non-invasive markers of disease activity may be used as an alternative to endoscopy to monitor disease response to therapy in patients with CD [30]. In addition, the presence of active inflammatory findings observed with radiology and ileocolonoscopy showed a moderate degree of agreement supporting our result. Especially, the degree of agreement was high when observing the anastomotic site. Park et al. [31] reported that a younger age at the time of diagnosis was significantly associated with CD-related surgery for Korean patients with CD (HR, 0.982; 95% CI, 0.966 to 0.998). In a prospective population-based cohort study, Solberg et al. [32] demonstrated that the need for systemic steroids after the establishment of the diagnosis was significantly associated with the development of advanced disease (odds ratio, 2.92; 95% CI, 1.18 to 7.21), supporting our results. Cross sectional image plus ileocolonoscopy may be more useful if the disease has a wide or advanced disease stage such as fistulating or stricturing disease, presence of perianal lesions, a history of previous intestinal resection surgery, or steroid use. Our research aimed to reduce unnecessary examinations according to the degree and conditions of individual diseases from the patient’s point of view rather than simply comparing examination methods.
There were several previous analyses reported as a tool for monitoring CD. Hara et al. [33] reported that imaging changes between CTE examinations have excellent potential for reliably monitoring the progression or regression of CD. Small-bowel CTE findings correlated well with endoscopy in a subset of patients (κ = 0.57; 95% CI, 0.20 to 0.94) [33]. Rimola et al. [34] found that the magnetic resonance index had high accuracy for the detection of disease activity (area under the receiver operating characteristic [ROC] curve 0.891, sensitivity 0.81, specificity 0.89). The calculated simplified magnetic resonance index of activity (MaRIA) also has a high (r = 0.81) and significant (p < 0.001) correlation with the Crohn’s disease endoscopic index of severity (CDEIS) of the corresponding segment in ileocolonic CD [34].
Our study has several clinical implications. First, compared with previously published reports on CD, our study had a relatively large sample size to help draw a conclusion. Second, we found that radiology plus ileocolonoscopy was not superior to radiology alone in predicting the prognosis of CD (p = 0.526, log-rank test). This could reduce the need for follow-up ileocolonoscopy when CTE or MRE is performed. CTE or MRE alone can save time and cost. Furthermore, when comparing the degree of agreement based on dividing by segments in the cases showing an active inflammatory state, we found a good agreement between CTE or MRE and ileocolonoscopy at the anastomotic site. Postoperatively in patients with CD at the anastomotic site, if there is a high degree of agreement between the two test methods, only one of the two tests can be performed and monitored. In particular, when the lesion is confined to the small intestine, radiology is required but not ileocolonoscopy. Third, based on our results, the presence of active inflammation observed with radiology and ileocolonoscopy was associated with clinical relapse. The possibility of clinically active inflammation can be predicted through the presence of active inflammation observed with radiology and/or ileocolonoscopy. In the future, follow-up studies on a non-invasive, inexpensive, and easy-to-repeat technique as a tool for monitoring CD are additionally needed.
The study has several limitations. First, all CTEs or MREs were interpreted by experienced radiologists, but no validated image scoring systems such as MaRIA, Clermont, or the London score were used [35–37]. Second, the FC was not included in this study because FC testing was not yet generalized due to the lack of coverage by the national reimbursement program in Korea. Insurance benefits for calprotectin testing were approved from April 2017. Finally, this study is also at risk for selection bias because patients in an active inflammatory state with worsening symptoms might have been more likely to have repeated radiologic or ileocolonoscopic examinations than patients who were in a remission state. Since the degree of active inflammation in the colon can be determined relatively easily by ileocolonoscopy, the group that was examined by ileocolonoscopy alone was not included. The results of this study should be carefully applied to patients with CD.
In conclusion, both radiology and ileocolonoscopy were not superior to radiology alone in predicting the prognosis of CD. The detection of active inflammation by radiology or ileocolonoscopy was associated with poor prognosis. Both the monitoring tools had a moderate degree of agreement in an active inflammatory state, especially at the anastomotic site. Therefore, tools for monitoring CD should be personalized according to the patient’s condition and additional tests should be carefully performed or avoided unless inclusive results are obtained on one test.
Figure 1
Flowchart showing the selection of the study population. CTE, computed tomography enterography; MRE, magnetic resonance enterography.
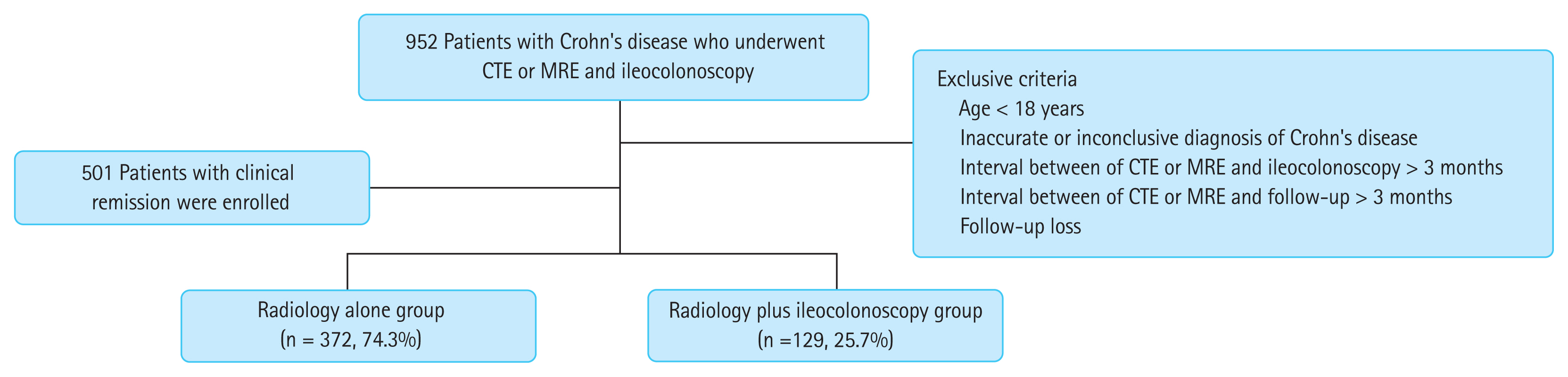
Figure 2
Cumulative incidence rates of relapse according to the presence of active inflammation in computed tomography enterography (CTE) or magnetic resonance enterography (MRE) and/or ileocolonoscopy.
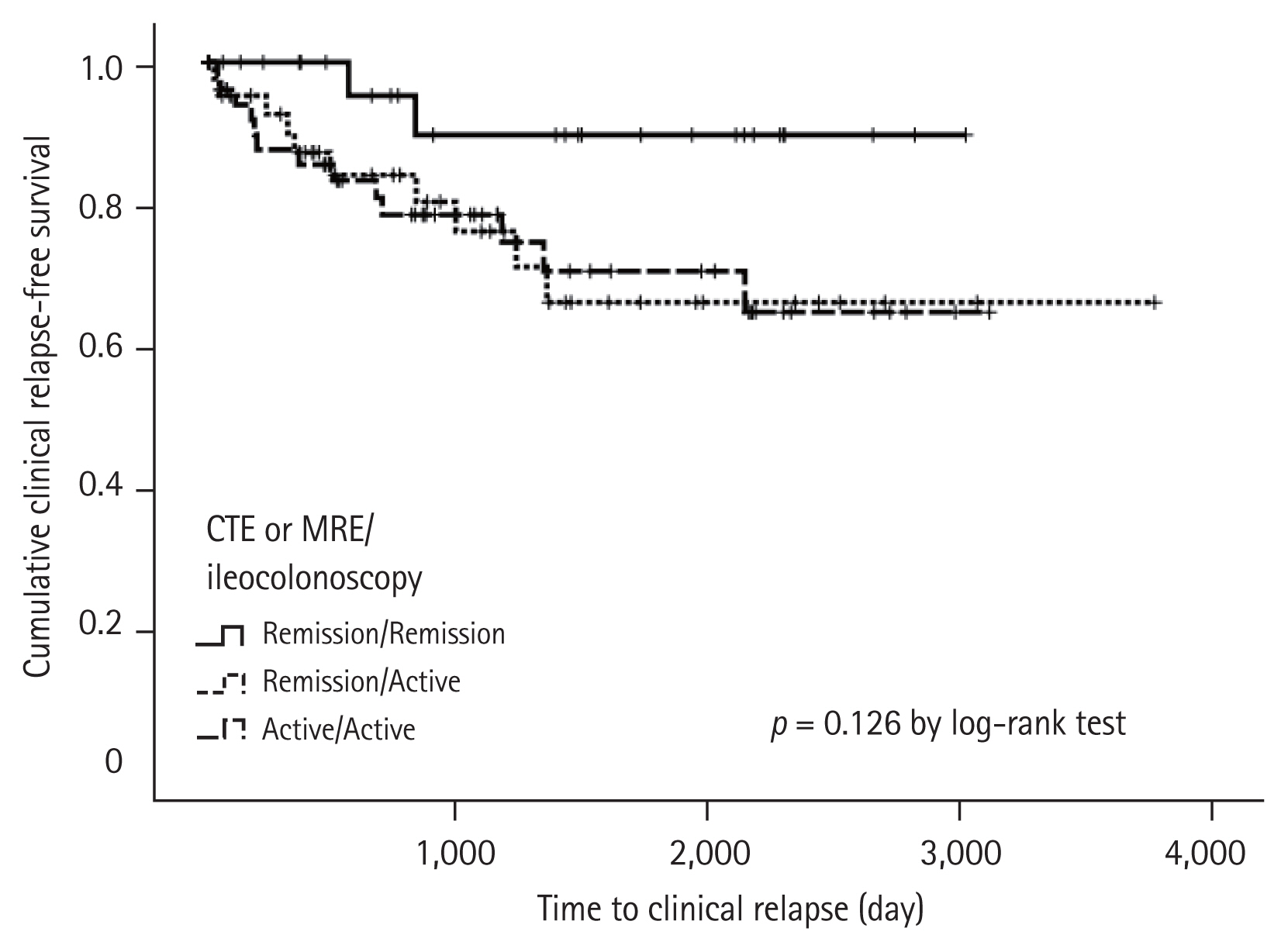
Figure 3
Cumulative incidence rates of relapse between computed tomography enterography (CTE) or magnetic resonance enterography (MRE) versus CTE or MRE plus ileocolonoscopy groups.
Table 1
Baseline characteristics of the study population
Table 2
Predictors of clinical relapse
| Variable | Univariate | Adjusting CTE (MRE) | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Multivariatea | ||||||
|
|
|
|||||
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Demographic variables | ||||||
| Age ≥ 40 years | Reference | |||||
| Age < 40 years | 2.985 | 1.381–6.451 | 0.005 | 2.756 | 1.263–6.013 | 0.011 |
| Male gender | 0.884 | 0.573–1.365 | 0.578 | 0.852 | 0.533–1.361 | 0.852 |
| Interval from diagnosis to CTE (MRE) | 1.000 | 1.000–1.000 | 0.220 | - | - | - |
| Montreal location | ||||||
| Ileal (L1) | Reference | |||||
| Colonic (L2) | 1.189 | 0.426–3.321 | 0.741 | - | - | - |
| Ileocolonic (L3) | 1.134 | 0.748–1.720 | 0.554 | - | - | - |
| Isolated upper GI disease (L4) | 1.205 | 0.165–8.816 | 0.854 | - | - | - |
| Montreal disease behavior | ||||||
| Nonstricturing, nonpenetrating (B1) | Reference | |||||
| Stricturing (B2) | 0.851 | 0.538–1.346 | 0.489 | 0.834 | 0.512–1.358 | 0.465 |
| Penetrating (B3) | 0.418 | 0.058–3.013 | 0.387 | 0.376 | 0.051–2.772 | 0.337 |
| Perianal disease modifier (p) | 1.158 | 0.768–1.746 | 0.485 | 1.078 | 0.683–1.700 | 0.748 |
| Previous intestinal resection surgery | 1.093 | 0.718–1.666 | 0.678 | 1.045 | 0.653–1.672 | 0.854 |
| Smoking status at diagnosis | ||||||
| Never smoked | Reference | |||||
| Current smoker | 0.880 | 0.441–1.758 | 0.718 | 1.013 | 0.494–2.077 | 0.971 |
| Ex-smoker | 0.730 | 0.365–1.459 | 0.373 | - | - | - |
| Current medication | ||||||
| Steroid | 2.300 | 1.421–3.722 | 0.000 | 2.121 | 1.258–3.577 | 0.005 |
| 5-ASA | 1.412 | 0.684–2.915 | 0.351 | - | - | - |
| Immunomodulator | 0.916 | 0.610–1.376 | 0.672 | - | - | - |
| Biologic monotherapy | 1.470 | 0.595–3.631 | 0.404 | - | - | - |
| Combination therapy | 1.024 | 0.447–2.344 | 0.956 | - | - | - |
| No medication | 1.147 | 0.466–2.828 | 0.765 | - | - | - |
| Laboratory variables | ||||||
| ESR ≤ 34 mm/hr | Reference | |||||
| ESR > 34 mm/hr | 0.949 | 0.628–1.434 | 0.804 | 0.742 | 0.463–1.188 | 0.215 |
| Serum CRP ≤ 11.5 mg/dL | Reference | |||||
| Serum CRP > 11.5 mg/dL | 1.327 | 0.833–2.113 | 0.233 | 1.239 | 0.729–2.107 | 0.428 |
| Serum albumin > 4.0 g/dL | Reference | |||||
| Serum albumin ≤ 4.0 g/dL | 1.440 | 0.957–1.440 | 0.080 | 1.295 | 0.816–2.056 | 0.273 |
| CTE (MRE) vs. Ileocolonoscopy | 1.160 | 0.732–1.839 | 0.527 | 1.316 | 0.799–2.168 | 0.281 |
Table 3
Comparison between patients who developed remission and those who did not
| Variable | Ileocolonoscopy | Kappa | p value | |
|---|---|---|---|---|
| Active (n = 126, 60.0%) | Remission (n = 84, 40.0%) | |||
| CTE (MRE) | ||||
| Active (n = 171, 81.4%) | 121 | 50 | 0.401 (−0.094 to 0.142) | < 0.001 |
| Remission (n = 39, 18.6%) | 5 | 34 | ||
Table 4
Comparison of colon segmental incidence of active lesions and agreement between CTE or MRE and ileocolonoscopy
REFERENCES
2. Baert F, Moortgat L, Van Assche G, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology 2010;138:463–468.


3. Colombel JF, Rutgeerts PJ, Sandborn WJ, et al. Adalimumab induces deep remission in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2014;12:414–422.


4. Landi B, Anh TN, Cortot A, et al. Endoscopic monitoring of Crohn’s disease treatment: a prospective, randomized clinical trial. The Groupe d’Etudes Therapeutiques des Affections Inflammatoires Digestives. Gastroenterology 1992;102:1647–1653.


5. Hilmi I, Kobayashi T. Capsule endoscopy in inflammatory bowel disease: when and how. Intest Res 2020;18:265–274.



6. Leighton JA, Shen B, Baron TH, et al. ASGE guideline: endoscopy in the diagnosis and treatment of inflammatory bowel disease. Gastrointest Endosc 2006;63:558–565.


7. Makkar R, Bo S. Colonoscopic perforation in inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2013;9:573–583.


8. Gale HI, Sharatz SM, Taphey M, Bradley WF, Nimkin K, Gee MS. Comparison of CT enterography and MR enterography imaging features of active Crohn disease in children and adolescents. Pediatr Radiol 2017;47:1321–1328.


9. Taylor SA. Editorial: can MRI enterography be an efficient tool for patient selection in clinical trials? Aliment Pharmacol Ther 2016;43:643–644.


10. da Silva Moraes AC, de Freitas Moraes G, de Araujo ALE, et al. Abdominal ultrasonography with color Doppler analysis in the assessment of ileal Crohn’s disease: comparison with magnetic resonance enterography. Intest Res 2019;17:227–236.



11. Makanyanga JC, Taylor SA. Current and future role of MR enterography in the management of Crohn disease. AJR Am J Roentgenol 2013;201:56–64.


12. Hordonneau C, Buisson A, Scanzi J, et al. Diffusion-weighted magnetic resonance imaging in ileocolonic Crohn’s disease: validation of quantitative index of activity. Am J Gastroenterol 2014;109:89–98.


13. Panes J, Bouhnik Y, Reinisch W, et al. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohns Colitis 2013;7:556–585.


14. Gomollon F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1. Diagnosis and medical management. J Crohns Colitis 2017;11:3–25.


15. Danese S, Sandborn WJ, Colombel JF, et al. Endoscopic, radiologic, and histologic healing with vedolizumab in patients with active Crohn’s disease. Gastroenterology 2019;157:1007–1018.


16. Guglielmo FF, Anupindi SA, Fletcher JG, et al. Small bowel Crohn disease at CT and MR enterography: imaging atlas and glossary of terms. Radiographics 2020;40:354–375.


17. Tolan DJ, Greenhalgh R, Zealley IA, Halligan S, Taylor SA. MR enterographic manifestations of small bowel Crohn disease. Radiographics 2010;30:367–384.


18. Elsayes KM, Al-Hawary MM, Jagdish J, Ganesh HS, Platt JF. CT enterography: principles, trends, and interpretation of findings. Radiographics 2010;30:1955–1970.


19. Bruining DH, Zimmermann EM, Loftus EV Jr, et al. Consensus recommendations for evaluation, interpretation, and utilization of computed tomography and magnetic resonance enterography in patients with small bowel Crohn’s disease. Radiology 2018;286:776–799.


20. Freeman HJ. Use of the Crohn’s disease activity index in clinical trials of biological agents. World J Gastroenterol 2008;14:4127–4130.



21. Kafil TS, Nguyen TM, MacDonald JK, Chande N. Cannabis for the treatment of Crohn’s disease. Cochrane Database Syst Rev 2018;11:CD012853.


22. Lydersen S. Cohen’s kappa: a measure of agreement between observers. Tidsskr Nor Laegeforen 2018;138:
https://doi.org/10.4045/tidsskr.17.0962
.

23. Serban ED. Treat-to-target in Crohn’s disease: will transmural healing become a therapeutic endpoint? World J Clin Cases 2018;6:501–513.



24. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015;110:1324–1338.



25. Bouguen G, Levesque BG, Feagan BG, et al. Treat to target: a proposed new paradigm for the management of Crohn’s disease. Clin Gastroenterol Hepatol 2015;13:1042–1050.


26. Van Assche G, Dignass A, Panes J, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. J Crohns Colitis 2010;4:7–27.


27. Buisson A, Gonzalez F, Poullenot F, et al. Comparative acceptability and perceived clinical utility of monitoring tools: a nationwide survey of patients with inflammatory bowel disease. Inflamm Bowel Dis 2017;23:1425–1433.


28. Siddiki HA, Fidler JL, Fletcher JG, et al. Prospective comparison of state-of-the-art MR enterography and CT enterography in small-bowel Crohn’s disease. AJR Am J Roentgenol 2009;193:113–121.


29. Nehra AK, Sheedy SP, Wells ML, et al. Imaging findings of ileal inflammation at computed tomography and magnetic resonance enterography: what do they mean when ileoscopy and biopsy are negative? J Crohns Colitis 2020;14:455–464.


30. Lopes S, Andrade P, Afonso J, et al. Monitoring Crohn’s disease activity: endoscopy, fecal markers and computed tomography enterography. Therap Adv Gastroenterol 2018;11:1756284818769075.



31. Park Y, Cheon JH, Park YL, et al. Development of a novel predictive model for the clinical course of Crohn’s disease: results from the CONNECT study. Inflamm Bowel Dis 2017;23:1071–1079.


32. Solberg IC, Cvancarova M, Vatn MH, Moum B. IBSEN Study Group. Risk matrix for prediction of advanced disease in a population-based study of patients with Crohn’s disease (the IBSEN Study). Inflamm Bowel Dis 2014;20:60–68.


33. Hara AK, Alam S, Heigh RI, Gurudu SR, Hentz JG, Leighton JA. Using CT enterography to monitor Crohn’s disease activity: a preliminary study. AJR Am J Roentgenol 2008;190:1512–1516.


34. Rimola J, Rodriguez S, Garcia-Bosch O, et al. Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn’s disease. Gut 2009;58:1113–1120.


35. Rozendorn N, Amitai MM, Eliakim RA, Kopylov U, Klang E. A review of magnetic resonance enterography-based indices for quantification of Crohn’s disease inflammation. Therap Adv Gastroenterol 2018;11:1756284818765956.



- TOOLS



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print



