 |
 |
| Korean J Intern Med > Volume 37(3); 2022 > Article |
|
Abstract
Background/Aims
The long-term effect of Helicobacter pylori eradication on the metabolic syndrome or diabetes are unclear. The aim of this study was to evaluate the effect of H. pylori eradication on glycemic control in type 2 diabetes mellitus (T2DM) or prediabetes mellitus (preDM).
Methods
A total of 124 asymptomatic subjects with T2DM or preDM were divided into H. pylori-negative (n = 40), H. pylori-positive with non-eradicated (n = 34), and eradicated (n = 50) groups. We measured H. pylori status (culture, histology, and rapid urease test) and glycated hemoglobin A1c (A1C) levels and followed-up at the 1st year and the 5th year of follow-up.
Results
The A1C levels significantly decreased in the eradicated group compared to the negative group and the non-eradicated groups (at the 1st year, p = 0.024; at the 5th year, p = 0.009). The A1C levels decreased in male, and/or subjects < 65 years of age in subgroup analyses (in male subjects, p = 0.047 and p = 0.020 at the 1st and the 5th year; in subjects < 65 years of age, p = 0.028 and p = 0.006 at the 1st and the 5th year; in male subjects < 65 years of age, p = 0.039 and p = 0.032 at the 1st and the 5th year). The eradication of H. pylori was related to the decrease in A1C values throughout the follow-up period, compared to the non-eradicated group (p = 0.017).
Helicobacter pylori is an important cause of gastritis and peptic ulcer diseases, and associated with diseases other than the gastrointestinal tract such as neurological disorders, cardiovascular diseases, asthma, immunologic impairment and nonalcoholic fatty liver disease [1]. Several studies have explained a relationship between H. pylori infection and insulin resistance (IR), metabolic syndrome (MS), diabetes mellitus (DM) [2–4], and considerable data support that H. pylori infection can induce pathogenic mechanisms of DM, such as IR and inflammation [5], that H. pylori infection is associated with the occurrence of DM and the level of glycated hemoglobin A1c (A1C) [6,7].
Recently, studies on the connection between the eradication of H. pylori and the improvement of metabolic parameters including A1C are being conducted, and several studies have reported decreases of fasting plasma glucose (FPG) and A1C levels after 3 or 6 months after H. pylori eradication [8–11]. However, the long-term effect of H. pylori eradication on the MS or DM has not been established yet. Thus, the aim of this study was to evaluate the long-term effect of H. pylori eradication on the glycemic control. As there are differences in sex and age in the incidence and development of MS and DM [12], additional subgroup analysis by sex and age was performed.
Total 124 asymptomatic subjects with type 2 diabetes mellitus (T2DM) or prediabetes mellitus (preDM) who underwent esophagogastroduodenoscopy (EGD) were selected and analyzed. They came from a cohort prospectively built for 5 years from May 2010 to February 2015 at the Seoul National University Bundang Hospital (SNUBH) (Fig. 1), after excluding the following criteria: (1) subjects with a history of gastric surgery and/or gastric cancer, esophageal cancer, type 1 DM, other major diseases including systemic inflammation or advanced malignant diseases; (2) subjects unsure whether they had previously received H. pylori eradication therapy; or (3) subjects whose A1C levels at baseline or at first year of follow-up are missing. Medical data of these subjects including sex, age, levels of FPG, A1C, total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), blood pressure (BP), and the prescription records (including changes) were collected using the Clinical Data Warehouse (CDW) in SNUBH, which has been widely used for medical research [13]. All participants completed a self-administration questionnaire under the supervision of the interviewer, which includes questions about smoking, alcohol consumption, height, weight. The Institutional Review Board (IRB) of SNUBH (B-1904-532-110) approved this study protocol. All subjects provided written informed consent in accordance with the ethical principles of the Declaration of Helsinki.
Trained nurses used a standardized protocol to obtain the anthropometric measurements, such as weight and height. Body mass index (BMI) value was calculated using the measured height and weight (kg/m2). BP was measured using an automated machine. Blood samples were collected in the morning after fasting overnight. The levels of A1C, TC, TG, LDL-C, HDL-C, and FPG were measured from the day before the administration of H. pylori eradication therapy in the eradicated group or the enrollment day in the H. pylori-negative and non-eradication groups using serum. We followed up each parameter at the 1 and 5 years after the eradication or enrollment dates.
T2DM and preDM were diagnosed on the basis of the ‘2019 Clinical practice guidelines for type 2 diabetes mellitus in Korea’ [14]. Hypertension and dyslipidemia were defined following The 2018 Guidelines by The Korean Society of Hypertension [15] and Korean Guidelines for the Management of Dyslipidemia 4th edition [16]. According to the revised International Diabetes Federation (IDF) definition, a person has MS if they have central obesity and any two of the following four factors: (1) raised BP: systolic blood pressure (SBP) ≥ 130 mmHg, diastolic blood pressure (DBP) ≥ 85 mmHg, or treatment of previously diagnosed hypertension; (2) reduced HDL-C levels: < 40 mg/dL in men and < 50 mg/dL in women; (3) raised TG levels: ≥ 150 mg/dL or specific treatment for abnormality in TG; and (4) FPG levels: > 100 mg/dL (> 5.6 mmol/L) or previously diagnosed T2DM. Generally, central obesity is defined as waist circumference > 90 cm in men and > 80 cm in women or BMI > 30 kg/m2. We defined central obesity as BMI > 25 kg/m2, according to the Korean Society for the Study of Obesity [17].
EGDs were done for diagnostic testing for H. pylori, and single endoscopist performed biopsies for consistency. Ten biopsy specimens were taken from the mid antrum and mid corpus for H. pylori testing: histology, culture, and rapid urease test. Four specimens of them were incubated for H. pylori at 37°C in microaerobic conditions for 3 to 5 days. Other four specimens were stained with modified Giemsa and hematoxylin and eosin stains, fixed in 10% neutral-buffered formalin, and embedded with paraffin to assess the presence of H. pylori. The others were used for Campylobacter-like organism (CLO) test or rapid urease test [18]. All biopsies were also examined by single expert pathologist for consistency.
Blood samples were taken from all subjects immediately after EGD. The serum was isolated and stored at −70°C until further evaluations. The samples were tested for immunoglobulin G (IgG) antibody against H. pylori using an enzyme-linked immunosorbent assay (Genedia H. pylori ELISA, Green Cross Medical Science Corp., Yongin, Korea), which has a sensitivity of 97.9% and specificity of 92.0% in the Korean population [19].
If any one of the three tests (culture, histology, or CLO test) had a positive result, the H. pylori infection was defined as a current infection. These current infection statuses were divided into H. pylori-positive with non-eradication and eradication group, according to whether or not H. pylori was eradicated. Positivity for the H. pylori serology test only and/or an H. pylori eradication history indicated a past H. pylori infection, which were excluded from this study. The H. pylori-negative group consisted of subjects with negative results for anti-H. pylori IgG antibodies as well as the histology, culture, and CLO tests.
The subjects with current H. pylori infection who wanted eradication therapy were treated with initial therapy, which consisted of triple therapy before 2012 [19], and a 10-day sequential therapy since then [19,20]. The triple therapy regimen was a combination of 40 mg of esomeprazole twice a day, 1,000 mg of amoxicillin twice a day, and 500 mg of clarithromycin twice a day for 7 days [19]. The 10-day sequential therapy included 40 mg of esomeprazol, 1,000 mg of amoxicillin twice a day for 5 days followed by 40 mg of esomeprazole twice a day, 500 mg of clarithromycin twice a day, and 500 mg of metronidazole twice a day for the next 5 days [20]. The H. pylori eradication was assessed using the 13C-urea breath test 4 weeks after treatment [19]. In subjects who failed the first-line regimen, we chose another 14-day quadruple regimen, containing bismuth (40 mg of esomeprazole twice a day, 300 mg of tripotassium dicitrate bismuthate [Denol, Greencross Co., Seoul, Korea] four times a day, 500 mg of metronidazole three times a day, and 500 mg of tetracycline four times a day), or a 14-day moxifloxacin-based triple therapy, containing 400 mg of moxifloxacin (Avelox, Bayer Health Care AG, Wuppertal, Germany) four times a day, 40 mg of esomeprazole twice a day, and 1,000 mg of amoxicillin twice a day [19]. At each follow-up visit for endoscopic surveillance, the status of H. pylori was evaluated using CLO test and/or biopsy. Therefore, it was confirmed that the H. pylori status was maintained during the follow-up period. The follow-up time points were classified as 1 and 5 years after eradication date in for the eradication group or enrollment dates for the other groups.
We performed the Pearson’s chi-square test for categorical variables and one-way analysis of variance (ANOVA) for continuous variables to compare baseline characteristics. Student’s t test and one-way ANOVA were used to compare the A1C levels and other metabolic parameters with respect to H. pylori status at each follow-up. To determine whether there was statistical significance in the A1C level changes, according to follow-up period differences among the groups, a linear mixed model was used. All analyses were performed using SPSS Windows version 22.0 (IBM Co., Armonk, NY, USA). If the p value was 0.05 or lower, the result was regarded as significant. And all statistical analysis and visualization process was conducted with consultation from the medical research collaborating center of SNUBH.
Among 124 subjects, the number of the H. pylori-negative (negative) group was 40, H. pylori-positive with no eradication (non-eradicated) group was 34, and H. pylori-positive with eradication (eradicated) group was 50 (Fig. 1). There were no significant differences in age, sex, the levels of A1C, TG, HDL-C, LDL-C, TC, SBP, BMI, FPG, the number of alcohol drinkers, smokers, subjects taking diabetes medication, subjects with T2DM, hypertension, hyperlipidemia and MS between groups (Table 1). DBP was lower in the eradication group than in the H. pylori-negative group (p = 0.035) (Table 1).
Primary eradication treatment was successful in 38 out of 50 patients (76%). Ten patients (20%) were successfully eradicated after secondary eradication, and finally, two (4%) patients were eradicated in three tries. During follow-up period, H. pylori tests were conducted along with endoscopy, and there was one case of re-infection after successful eradication. The case of re-infection was excluded from the analysis.
The improvement in A1C was statistically significant after the eradication of H. pylori at the 1st and the 5th year of follow-up. The A1C level was significantly decreased in the eradicated group compared to the non-eradicated group (1st year, p = 0.025; 5th year, p = 0.012), and to the other two groups (1st year, p = 0.024; 5th year, p = 0.009) (Table 2). No one had converted to normal after H. pylori eradication in 42 T2DM patients, and hemoglobin A1c (HbA1c) level of one of eight prediabetes patients became normal after eradication.
Additionally, we observed a correlation between the follow-up period and group differences after excluding adjusted variables, such as age, sex, smoking, alcohol consumption, hypertension, and dyslipidemia, using a linear mixed model. Table 3 shows the results, with age and follow-up time identified as independent factors associated with increases in A1C (age, p = 0.002; time, p = 0.014), while dyslipidemia as an independent factor associated with decreases in A1C (dyslipidemia, p = 0.028). In the analysis of the relation between time and H. pylori status, a decrease in HbA1c was observed over time in eradicated group compared to non-eradicated group (time & eradicated, p = 0.017). The result of the analysis by dividing the follow-up time periodically (from the baseline to the 1st year of follow-up, and from the 1st year to the 5th year of follow-up) also showed statistically significant decreases in A1C (1st year, p = 0.028; 5th year, p = 0.006) (Fig. 2A).
In the subgroup analysis with respect to age and sex, A1C values were decreased in the subjects who were male or under 65 years of age in the eradicated group (Table 4). In men, A1C levels decreased in the eradicated group compared to the non-eradicated group (1st year, p = 0.023; 5th year, p = 0.007), and to the other two groups (1st year, p = 0.047; 5th year, p = 0.020). In patients under 65 years of age, A1C levels decreased in the eradicated group compared to the non-eradicated group (1st year, p = 0.012; 5th year, p = 0.009) (Table 4), and to the other two groups (1st year, p = 0.028; 5th year, p = 0.006). Moreover, male subjects aged < 65 years showed statistically significant improvement in A1C values in the eradicated group compared to the non-eradication group (1st year, p = 0.008; 5th year, p = 0.007), and to the other two groups (1st year, p = 0.039; 5th year, p = 0.032). In contrast, the changes in A1C values were not statistically different between the three groups in female subjects or age above 65 years (all p > 0.05), although there was a decreasing tendency of A1C level after eradication (Table 4).
We had confirmed the administered medications in subjects. There were 10 and 25 subjects in each 1- and 5-year follow-up group who changed diabetes medication (Supplementary Table 1), which could affect the A1C levels. Even after excluding these subjects, the A1C level was significantly decreased in the eradicated group compared to the non-eradicated group (1st year, p = 0.006; 5th year, p = 0.001), and to the other two groups (1st year, p = 0.005; 5th year, p = 0.002) (Supplementary Table 2). This result was consistent in the linear mixed model which observed a correlation between the follow-up period and group differences after excluding adjusted variables (1st year, p = 0.009; 5th year, p = 0.002) (Fig. 2B).
There were no significant differences in the prescription of statins and proton pump inhibitors (PPIs) between groups, as 27 patients out of 40 H. pylori-negative patients (67.5%), 27 patients out of 34 non-eradicated patients (79.4%), and 38 out of 50 eradicated patients (74%) were found to have been prescribed statins, and 36 out of 40 H. pylori-negative patients (90%), 30 out of 34 non-eradicated patients (79.4%) and 49 out of 50 eradicated patients (98%) were found to have been prescribed PPIs, except for the eradication regimen.
Although metabolic parameters did not display statistically significant differences between the three groups (all p > 0.05), FPG showed a tendency to improve in the eradicated group compared to the other two groups. Similarly, BMI at the 5th year of follow-up, SBP and DBP at the 1st year of follow-up showed tendencies to improve (Supplementary Table 3). In female subjects at the 5th year of follow-up, BMI significantly decreased in the eradicated group compared to the non-eradicated group (p = 0.014), and to the other two groups (p = 0.027), and SBP also decreased in the eradicated group compared to the non-eradicated group (p = 0.020) (Supplementary Table 4). The prevalence of MS has been observed to decrease at the 1st year of follow-up, but with no statistical significance (odds ratio, 1.34; p = 0.535) (Supplementary Table 5).
In this long-term follow-up study, we found that H. pylori eradication was related to the decreasing of A1C levels in patients with T2DM or preDM, especially in male and subjects under 65 years of age. To the best of our knowledge, this is the first suggestion of a positive association between H. pylori eradication and improvement in the A1C level in patients with T2DM or preDM over a long period.
This finding is consistent with the results of other studies on the effectiveness of H. pylori eradication over a short period [8,9,21]. As mentioned previously, Dogan et al. [10] reported significant changes of FPG and A1C level between baseline and 6 months after H. pylori eradication in subjects with normal glucose levels. In addition, in 319 patients with T2DM, Cheng et al. [11] demonstrated that the eradicated group had significant within-group and between-group improvements in A1C, compared with the non-eradicated group. Although the exact mechanism remains unknown, researchers have hypothesized potential mechanisms linking H. pylori infection to metabolic parameters. Regarding the pathogenesis proposed to associate H. pylori infection with atherogenic dyslipidemia, experimental investigations have demonstrated that chronic bacterial infections, such as an H. pylori infection, induces the production of proinflammatory cytokines. These cytokines, such as interleukin 1 (IL-1) and IL-6, interferon-α, and tumor necrosis factor-α, can alter lipid metabolism in variety of ways. It also reduces the activation of adipose tissue lipoprotein lipase, triggers hepatic fatty acid synthesis, influences lipolysis, and stimulates 3-hydroxy-3-methylglutaryl-coenzyme A reductase activity in the liver [22]. An H. pylori infection possibly be associated with impaired hepatic IR through the c-Jun/microRNA-203/suppressor of the cytokines that signal three pathway [23]. In addition, inflammatory cytokines induce the phosphorylation of serine/threonine residues in the insulin receptor, preventing its activation and reducing insulin sensitivity [24]. Ghrelin is involved in ingestion, appetite, and nutrition, especially lipid absorption and lipogenesis [25]. It can also regulate insulin sensitivity and stimulate insulin-induced glucose uptake [26], and an H. pylori infection can impair its synthesis [27]. Ghrelin was significantly lower in H. pylori positive than in H. pylori-negative individuals [28]. Low plasma ghrelin levels are associated with elevated FPG levels and IR [29]. Previously, we found that H. pylori eradication upregulates preproghrelin mRNA expression and increases plasma acyl ghrelin levels, contributing to the abatement of postprandial distress symptoms in functional dyspepsia [30]. Another possible explanation involves glucagon-like peptide-1 (GLP-1) and the gut microbiota. GLP-1 regulates glucose homeostasis [31], has trophic effects on pancreatic beta cells [32], reduces acid secretion in the stomach, and delays gastric emptying. It also regulates appetite by inducing a feeling of satiety. Specific bacteria, including Bifidobacterium spp., Lactobacillus spp., and Akkermansia muciniphila spp., contribute to the regulation of incretin hormones such as GLP-1 [33]. A conventional eradication treatment could improve carbohydrate metabolism by increasing GLP-1 secretion, and this change is closely related to alterations in the gut microbiota [8,34], even after a long-term follow-up [35]. These underlying mechanisms support our observation of the effect of H. pylori eradication on the decrease of in A1C levels over a long-term follow-up.
An interesting result of this study is that the positive association between the eradication of H. pylori and A1C levels appeared in patients < 65 years of age. This positive association in younger age is consistent with the results of earlier research on the relationship between H. pylori infection and MS. Chen et al. [36] observed that subjects with MS < 50 years of age had a higher prevalence of H. pylori infection. Similarly, our team demonstrated that the association between H. pylori seropositivity and MS was present in subjects < 65 years of age but disappeared in the older ones [12]. Park et al. [37] reported that the effect of H. pylori eradication on the risk of dyslipidemia was more evident in subjects < 50 years of age. Altogether MS prevalence was not significantly different regardless of the H. pylori serological status in older subject, they tend to have other chronic diseases, such as hypertension, hyperlipidemia, or diabetes, contributing to the development of MS. Regardless of geographic location, the H. pylori infection in most individuals is acquired during early childhood [38]. This chronic H. pylori infection plays an important role in the development of development in the adulthood but this systemic inflammation caused by H. pylori infection may influence MS development somewhat at an early stage, but not in an advanced stage or old age [39]. Thus early eradication of H. pylori is more beneficial in terms of prevention of MS.
Our study also suggested a correlation between sex and A1C levels, specifically in male subjects. These results are compatible with previous studies that showed male subjects are more affected by H. pylori infection than female subjects, such as higher gastric inflammation and activity scores in the antrum with H. pylori infection [40]. According to a large dataset including worldwide mortality and prevalence of cancers, the incidence of gastric cancer in male subjects was about twice as high as in female individuals [41]. Interestingly, in a mouse-model study, severe dysplasia and gastric carcinogenesis predominated in male subjects infected with H. pylori [42]. Furthermore, MS was higher in male subjects, except in the age-group of > 70 years, and the MS in female subjects after definite menopause was higher than in male subjects [12]. Similarly, the H. pylori infection increased the risk of MS in female subjects aged ≥ 50 years [43]. Furthermore, H. pylori infection was a statistically significant predictor of DM incidence in male subjects [6]. The underlying mechanism is related to estrogen. Ovarian-dependent female hormones, such as estradiol (E2), prevent gastric carcinogenesis and inflammation of the gastric tissue in insulin–gastrin mice infected with H. pylori. In addition, exogenous E2 supplementation have shown protective effects against the development of H. pylori-induced gastritis and premalignant lesions through several mechanisms [44]. In the relationship between MS and E2, recent researches showed that TC, LDL-C, and TG were higher in postmenopausal female subjects than in premenopausal females [45]. In addition, hormone therapy with estrogen alone has been associated with decreased TC and LDL-C levels and elevated HDL-C levels [46]. Considering these, we speculate that the difference in the association of H. pylori infection with DM in our study might be related to sex hormone-induced inflammation.
As our study was a retrospective study from a cohort that selected prospectively for 12 years, the number of subjects became small due to exclusion of missing data among metabolic parameters. Small number of subjects that can weaken the conclusion is one of the limitations of this study. However, the patient selection was not biased, and we collected the data on levels of A1C, TC, TG, LDL-C, HDL-C, FPG, BP and the duration and changes of diabetes medication in each subject from the CDW of SNUBH as well as a medical cohort, which were well maintained. We excluded serologic positivity in the H. pylori-negative group, and thus, chronic inflammation can be reliably excluded in that group. Most previous studies compared only before and after treatment in the H. pylori-infected group without a control group with short-term follow-up period. In our study, subjects were divided into H. pylori-negative, H. pylori-positive with non-eradication, and H. pylori-positive with eradication groups, which allowed us to reliably determine the independent effect of H. pylori eradication compared to the other groups. Due to the nature of DM, it is difficult to observe without drug changes during long-term follow-up. In this study, insulin-based patients were excluded to compensate for this limitation, and patients who changed medication during the observation period showed no significant difference with 17.5% in the negative group, 14.7% in the non-eradicated group and 26% in the eradicated group. And additional analyses were conducted after excluding patients who changed oral medications to confirm that the same results were maintained.
IR can be evaluated using homeostasis model assessment of insulin resistance (HOMA-IR), which uses the fasting insulin level with the fasting glucose level. In our study, the fasting insulin level was not measured in all subjects, so HOMA-IR was unable to be calculated. Future study about the effect of H. pylori eradication in IR using HOMA-IR would be needed.
Despite the statistical significance of H. pylori eradication in A1C and some improvement tendency in FPG, BMI, SBP, and DBP, we have failed to show a correlation between the three groups in other metabolic parameters such as TC, TG, LDL-C, HDL-C. It might be explained that, because we selected a study group in patients with T2DM or prediabetes, results may differ from studies in the dyslipidemia group or the normal lipid group. Also studies have been conducted with a relatively small number of subjects, it may not be possible to determine the effectiveness of eradication therapy in dyslipidemia. Therefore, a large-scale study is needed to determine the effect of H. pylori treatment in patients with dyslipidemia in the future.
In conclusion, H. pylori eradication was related to the decreasing of A1C levels over a long-term follow-up period, especially in male subjects and those < 65 years of age. Our study provides new insights into the role of H. pylori eradication and supports the rationale for recommendation of H. pylori eradication therapy in male or young subjects with T2DM or preDM.
Acknowledgments
This work was supported by grant (no. 06-2020-184) from the Seoul National University Bundang Hospital Research fund. In addition, this work was supported by a grant (2011-0030001) from the National Research Foundation (NRF) for the Global Core Research Center (GCRC) funded by the Ministry of Science, ICT and Future Planning (MSIP), Republic of Korea.
The authors thank Division of Statistics in Medical Research Collaborating Center at Seoul National University Bundang Hospital for statistical analysis.
Figure 1
Study flow chart. Totally 124 subjects were selected and divided into three groups: Helicobacter pylori-negative, non-eradicated, eradicated. Eighty-seven subjects were followed up for up to 5 years.
Figure 2
Hemoglobin A1c (HbA1c) changes over time in each group. (A) Analysis of the entire subjects and (B) analysis except for patients who had hypoglycemic agent changes during follow-up (FU). Mean HbA1c (A1C) values of each group are demonstrated. Improvements in A1C after Helicobacter pylori eradication at the 1st year of FU were observed, and these changes were maintained until the 5th year of FU, in both (A) and (B). The p values were calculated using linear mixed model. Adjusted variables; age, sex, smoke, alcohol consumption, hypertension, and dyslipidemia. aAdjusted p value, at the 1st year of FU, bAdjusted p value, at the 5th year of FU.
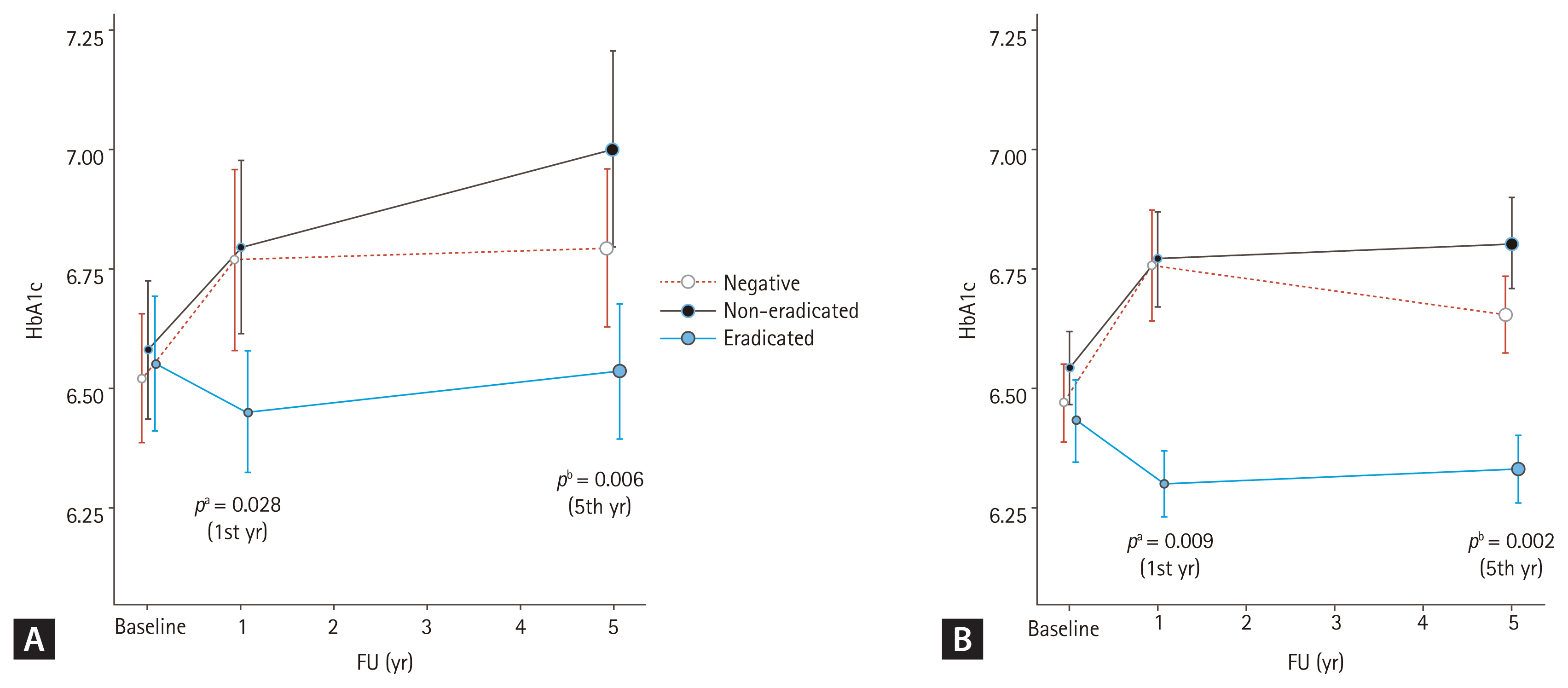
Table 1
Demographic and baseline characteristics of included subjects according to Helicobacter pylori status
| Characteristic | Total (n = 124) | H. pylori-negative (n = 40) | H. pylori-positive | p valuea | |
|---|---|---|---|---|---|
| Non-eradicated (n = 34) | Eradicated (n = 50) | ||||
| Age, yr | 61.43 ± 10.45 | 61.13 ± 10.14 | 62.82 ± 12.60 | 60.72 ± 9.14 | 0.651 |
| Age < 65 yr | 71 (57.3) | 25 (62.5) | 16 (47.1) | 30 (60.0) | 0.359 |
| Male sex | 72 (59.0) | 21 (52.5) | 24 (70.6) | 27 (55.6) | 0.219 |
| Current/ex-smoker | 59 (47.6) | 17 (42.5) | 20 (58.8) | 22 (44.0) | 0.302 |
| Current/ex-drinker | 75 (61.5) | 21 (52.5) | 22 (64.7) | 32 (64.0) | 0.454 |
| T2DM | 104 (83.9) | 31 (77.5) | 31 (91.2) | 42 (84.0) | 0.281 |
| Prediabetes | 20 (16.1) | 9 (22.5) | 3 (8.8) | 8 (16.0) | |
| Diabetes medication | 66 (51.6) | 20 (50.0) | 15 (44.1) | 24(48.0) | 0.878 |
| Metformin | 44 (35.5) | 13 (32.5) | 13 (38.2) | 18 (36.0) | 0.872 |
| Sulfonylurea | 34 (27.4) | 12 (30.0) | 8 (23.5) | 14 (28.0) | 0.818 |
| Othersb | 30 (24.2) | 8 (20.0) | 8 (23.5) | 14 (28.0) | 0.609 |
| Hypertension | 82 (66.1) | 28 (70.0) | 22 (64.7) | 32 (64.0) | 0.819 |
| Hyperlipidemia | 64 (51.6) | 17 (34.0) | 21 (61.8) | 26 (52.0) | 0.255 |
| A1C, % | 6.55 ± 0.08 | 6.52 ± 0.13 | 6.58 ± 0.14 | 6.55 ± 0.14 | 0.961 |
| TG, mg/dL | 150.52 ± 7.85 | 148.97 ± 15.42 | 162.89 ± 16.48 | 143.79 ± 10.71 | 0.609 |
| HDL-C, mg/dL | 48.30 ± 1.19 | 49.34 ± 2.63 | 49.56 ± 2.20 | 46.73 ± 1.58 | 0.536 |
| LDL-C, mg/dL | 100.11 ± 2.67 | 105.78 ± 4.93 | 100.81 ± 4.94 | 94.70 ± 4.04 | 0.215 |
| TC, mg/dL | 179.76 ± 3.77 | 188.69 ± 6.21 | 181.44 ± 8.42 | 172.08 ± 5.34 | 0.173 |
| SBP, mmHg | 134.84 ± 2.19 | 135.57 ± 4.63 | 133.73 ± 3.12 | 135.00 ± 3.33 | 0.946 |
| DBP, mmHg | 76.96 ± 1.12 | 80.19 ± 1.83 | 77.43 ± 2.27 | 73.45 ± 1.64 | 0.035c |
| BMI, kg/m2 | 25.24 ± 0.30 | 25.29 ± 0.44 | 25.57 ± 0.69 | 24.97 ± 0.45 | 0.708 |
| FPG, mg/dL | 130.44 ± 4.33 | 128.00 ± 8.14 | 135.35 ± 7.67 | 128.68 ± 6.86 | 0.763 |
| MS | 61 (50.0) | 18 (46.2) | 18 (55.0) | 25 (50.0) | 0.777 |
T2DM, type 2 diabetes mellitus; A1C, glycated hemoglobin A1c; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; FPG, fasting plasma glucose; MS, metabolic syndrome.
a Student’s t test and analysis of variance (ANOVA) were used for continuous data, and chi-square test was used for categorical variables.
Table 2
Changes in glycated hemoglobin A1c depending on Helicobacter pylori status at each time point of follow-up compared to baseline
| Variable | H. pylori-negative | H. pylori-positive | p valueb | ||
|---|---|---|---|---|---|
| Non-eradicated | Eradicated | p valuea | |||
| 1-year follow-up (n = 124) | 0.25 ± 0.12 (n = 40) | 0.21 ± 0.11 (n = 34) | −0.10 ± 0.09 (n = 50) | 0.025c | 0.024c |
| 5-year follow-up (n = 87) | 0.34 ± 0.15 (n = 25) | 0.29 ± 0.12 (n = 25) | −0.13 ± 0.11 (n = 37) | 0.012c | 0.009c |
Table 3
Multivariate analysis regarding changes in glycated hemoglobin A1c depending on Helicobacter pylori status
| Variable | Estimate | p valuea |
|---|---|---|
| Current/ex-smoker (reference, non-smoker) | 0.09 ± 0.20 | 0.430 |
| Current/ex-drinker (reference, non-drinker) | −0.14 ± 0.15 | 0.177 |
| Hypertension (reference, normal) | −0.08 ± 0.14 | 0.649 |
| Dyslipidemia (reference, normal) | −0.35 ± 0.14 | 0.028b |
| Age (increasing) | 0.03 ± 0.01 | 0.002b |
| Male (reference, female) | 0.23 ± 0.21 | 0.182 |
| H. pylori status (reference, non-eradicated) | ||
| Negative | 0.06 ± 0.18 | 0.952 |
| Eradicated | −0.06 ± 0.17 | 0.672 |
| Timec | 0.07 ± 0.02 | 0.014b |
| Time & H. pylori status (negative)c | −0.01 ± 0.03 | 0.805 |
| Time & H. pylori status (eradicated)c | −0.08 ± 0.03 | 0.017b |
Table 4
Changes in glycated hemoglobin A1c depending on age, sex and Helicobacter pylori status at each time point of follow-up time compared to baseline
| Variable | H. pylori-negative | H. pylori-positive | p valueb | ||
|---|---|---|---|---|---|
| Non-eradicated | Eradicated | p valuea | |||
| Age < 65 years | |||||
| 1 year (n = 71) | 0.30 ± 0.17 | 0.21 ± 0.08 | −0.12 ± 0.08 | 0.012c | 0.028c |
| 5 years (n = 51) | 0.50 ± 0.77 | 0.40 ± 0.15 | −0.11 ± 0.56 | 0.009c | 0.006c |
| Age ≥ 65 years | |||||
| 1 year (n = 53) | 0.15 ± 0.14 | 0.22 ± 0.20 | −0.07 ± 0.18 | 0.284 | 0.464 |
| 5 years (n = 36) | 0.25 ± 0.19 | 0.19 ± 0.18 | −0.12 ± 0.21 | 0.271 | 0.500 |
| Male | |||||
| 1 year (n = 72) | −0.02 ± 0.11 | 0.20 ± 0.14 | −0.26 ± 0.13 | 0.023c | 0.047c |
| 5 years (n = 49) | 0.21 ± 0.22 | 0.26 ± 0.12 | −0.25 ± 0.14 | 0.007c | 0.020c |
| Female | |||||
| 1 year (n = 52) | 0.54 ± 0.19 | 0.25 ± 0.16 | 0.08 ± 0.09 | 0.351 | 0.072 |
| 5 years (n = 38) | 0.45 ± 0.20 | 0.36 ± 0.27 | 0.05 ± 0.18 | 0.327 | 0.297 |
| Male & < 65 years | |||||
| 1 year (n = 45) | −0.02 ± 0.15 | 0.25 ± 0.10 | −0.22 ± 0.12 | 0.008c | 0.039c |
| 5 years (n = 32) | 0.33 ± 0.26 | 0.47 ± 0.16 | −0.12 ± 0.14 | 0.007c | 0.032c |
| Male & ≥ 65 years | |||||
| 1 year (n = 27) | −0.01 ± 0.17 | 0.15 ± 0.30 | −0.33 ± 0.33 | 0.298 | 0.492 |
| 5 years (n = 17) | −0.10 ± 0.38 | −0.04 ± 0.12 | −0.50 ± 0.30 | 0.184 | 0.371 |
| Female & < 65 years | |||||
| 1 year (n = 26) | 0.71 ± 0.29 | 0.07 ± 0.07 | 0.03 ± 0.08 | 0.853 | 0.058 |
| 5 years (n = 19) | 0.66 ± 0.27 | 0.05 ± 0.25 | −0.10 ± 0.24 | 0.775 | 0.122 |
| Female & ≥ 65 years | |||||
| 1 year (n = 26) | 0.30 ± 0.21 | 0.33 ± 0.22 | 0.08 ± 0.18 | 0.510 | 0.754 |
| 5 years (n = 19) | 0.10 ± 0.23 | 0.47 ± 0.35 | 0.20 ± 0.26 | 0.543 | 0.685 |
REFERENCES
1. Lim JH, Kim N, Lim SH, et al. Inverse relationship between Helicobacter pylori infection and asthma among adults younger than 40 years: a cross-sectional study. Medicine (Baltimore) 2016;95:e2609.


2. Gunji T, Matsuhashi N, Sato H, et al. Helicobacter pylori infection is significantly associated with metabolic syndrome in the Japanese population. Am J Gastroenterol 2008;103:3005–3010.


3. Polyzos SA, Kountouras J, Zavos C, Deretzi G. The association between Helicobacter pylori infection and insulin resistance: a systematic review. Helicobacter 2011;16:79–88.


4. Simon L, Tornoczky J, Toth M, Jambor M, Sudar Z. The significance of Campylobacter pylori infection in gastroenterologic and diabetic practice. Orv Hetil 1989;130:1325–1329.

5. Kayar Y, Pamukcu O, Eroglu H, Kalkan Erol K, Ilhan A, Kocaman O. Relationship between Helicobacter pylori infections in diabetic patients and inflammations, metabolic syndrome, and complications. Int J Chronic Dis 2015;2015:290128.




6. Chen YY, Fang WH, Wang CC, et al. Helicobacter pylori infection increases risk of incident metabolic syndrome and diabetes: a cohort study. PLoS One 2019;14:e0208913.



7. Bajaj S, Rekwal L, Misra SP, Misra V, Yadav RK, Srivastava A. Association of helicobacter pylori infection with type 2 diabetes. Indian J Endocrinol Metab 2014;18:694–699.



8. Cornejo-Pareja I, Martin-Nunez GM, Roca-Rodriguez MM, et al. H. pylori eradication treatment alters gut microbiota and GLP-1 secretion in humans. J Clin Med 2019;8:451.



9. Martin-Nunez GM, Cornejo-Pareja I, Coin-Araguez L, et al. H. pylori eradication with antibiotic treatment causes changes in glucose homeostasis related to modifications in the gut microbiota. PLoS One 2019;14:e0213548.



10. Dogan Z, Sarikaya M, Ergul B, Filik L. The effect of Helicobacter pylori eradication on insulin resistance and HbA1c level in people with normal glucose levels: a prospective study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2015;159:242–245.


11. Cheng KP, Yang YJ, Hung HC, et al. Helicobacter pylori eradication improves glycemic control in type 2 diabetes patients with asymptomatic active Helicobacter pylori infection. J Diabetes Investig 2019;10:1092–1101.




12. Lim SH, Kim N, Kwon JW, et al. Positive association between Helicobacter pylori infection and metabolic syndrome in a Korean population: a multicenter nationwide study. Dig Dis Sci 2019;64:2219–2230.



13. Yoo S, Lee KH, Lee HJ, et al. Seoul National University Bundang Hospital’s electronic system for total care. Healthc Inform Res 2012;18:145–152.



14. Kim MK, Ko SH, Kim BY, et al. 2019 Clinical practice guidelines for type 2 diabetes mellitus in Korea. Diabetes Metab J 2019;43:398–406.




15. Kim KI, Ihm SH, Kim GH, et al. 2018 Korean Society of Hypertension guidelines for the management of hypertension: part III-hypertension in special situations. Clin Hypertens 2019;25:19.




16. Rhee EJ, Kim HC, Kim JH, et al. 2018 Guidelines for the management of dyslipidemia. Korean J Intern Med 2019;34:723–771.




17. Seo MH, Lee WY, Kim SS, et al. 2018 Korean Society for the Study of Obesity guideline for the management of obesity in Korea. J Obes Metab Syndr 2019;28:40–45.



18. Kim SE, Park YS, Kim N, et al. Effect of Helicobacter pylori eradication on functional dyspepsia. J Neurogastroenterol Motil 2013;19:233–243.



19. Lee JY, Kim N, Kim MS, et al. Factors affecting first-line triple therapy of Helicobacter pylori including CYP2C19 genotype and antibiotic resistance. Dig Dis Sci 2014;59:1235–1243.



20. Huang J, Zhou L, Geng L, et al. Randomised controlled trial: sequential vs. standard triple therapy for Helicobacter pylori infection in Chinese children: a multicentre, open-labelled study. Aliment Pharmacol Ther 2013;38:1230–1235.


21. Mokhtare M, Mirfakhraee H, Arshad M, et al. The effects of helicobacter pylori eradication on modification of metabolic syndrome parameters in patients with functional dyspepsia. Diabetes Metab Syndr 2017;11(Suppl 2):S1031–S1035.


22. Feingold KR, Grunfeld C. Role of cytokines in inducing hyperlipidemia. Diabetes 1992;41(Suppl 2):97–101.



23. Zhou X, Liu W, Gu M, Zhou H, Zhang G. Helicobacter pylori infection causes hepatic insulin resistance by the c-Jun/miR-203/SOCS3 signaling pathway. J Gastroenterol 2015;50:1027–1040.



24. Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest 2005;115:1111–1119.



25. Chabot F, Caron A, Laplante M, St-Pierre DH. Interrelationships between ghrelin, insulin and glucose homeostasis: physiological relevance. World J Diabetes 2014;5:328–341.



26. Patel AD, Stanley SA, Murphy KG, et al. Ghrelin stimulates insulin-induced glucose uptake in adipocytes. Regul Pept 2006;134:17–22.


27. Paoluzi OA, Blanco del VG, Caruso R, Monteleone I, Monteleone G, Pallone F. Impairment of ghrelin synthesis in Helicobacter pylori-colonized stomach: new clues for the pathogenesis of H. pylori-related gastric inflammation. World J Gastroenterol 2014;20:639–646.



28. Gao XY, Kuang HY, Liu XM, Duan P, Yang Y, Ma ZB. Circulating ghrelin/obestatin ratio in subjects with Helicobacter pylori infection. Nutrition 2009;25:506–511.


29. Poykko SM, Kellokoski E, Horkko S, Kauma H, Kesaniemi YA, Ukkola O. Low plasma ghrelin is associated with insulin resistance, hypertension, and the prevalence of type 2 diabetes. Diabetes 2003;52:2546–2553.



30. Choi YJ, Kim N, Yoon H, et al. Increase in plasma acyl ghrelin levels is associated with abatement of dyspepsia following Helicobacter pylori eradication. J Gastroenterol 2016;51:548–559.



31. Rask E, Olsson T, Soderberg S, et al. Impaired incretin response after a mixed meal is associated with insulin resistance in nondiabetic men. Diabetes Care 2001;24:1640–1645.



33. Zietek T, Rath E. Inflammation meets metabolic disease: gut feeling mediated by GLP-1. Front Immunol 2016;7:154.



34. Ianiro G, Tilg H, Gasbarrini A. Antibiotics as deep modulators of gut microbiota: between good and evil. Gut 2016;65:1906–1915.


35. Liou JM, Chen CC, Chang CM, et al. Long-term changes of gut microbiota, antibiotic resistance, and metabolic parameters after Helicobacter pylori eradication: a multicentre, open-label, randomised trial. Lancet Infect Dis 2019;19:1109–1120.


36. Chen LW, Chien CY, Yang KJ, Kuo SF, Chen CH, Chien RN. Helicobacter pylori infection increases insulin resistance and metabolic syndrome in residents younger than 50 years old: a community-based study. PLoS One 2015;10:e0128671.



37. Park Y, Kim TJ, Lee H, et al. Eradication of Helicobacter pylori infection decreases risk for dyslipidemia: a cohort study. Helicobacter 2021;26:e12783.



38. Buzas GM. Metabolic consequences of Helicobacter pylori infection and eradication. World J Gastroenterol 2014;20:5226–5234.



39. Pai JK, Pischon T, Ma J, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med 2004;351:2599–2610.


40. el-Omar EM, Penman ID, Ardill JE, Chittajallu RS, Howie C, McColl KE. Helicobacter pylori infection and abnormalities of acid secretion in patients with duodenal ulcer disease. Gastroenterology 1995;109:681–691.


41. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69–90.


42. Ohtani M, Ge Z, Garcia A, et al. 17β-Estradiol suppresses Helicobacter pylori-induced gastric pathology in male hypergastrinemic INS-GAS mice. Carcinogenesis 2011;32:1244–1250.



43. Yu Y, Cai J, Song Z, Wang J, Wu L. Association of Helicobacter pylori infection with metabolic syndrome in aged Chinese females. Exp Ther Med 2019;17:4403–4408.



44. Ohtani M, Garcia A, Rogers AB, et al. Protective role of 17 beta-estradiol against the development of Helicobacter pylori-induced gastric cancer in INS-GAS mice. Carcinogenesis 2007;28:2597–2604.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print



