 |
 |
| Korean J Intern Med > Volume 37(2); 2022 > Article |
|
Abstract
Background/Aims
Data comparing the antibody responses of different coronavirus disease 2019 (COVID-19) vaccine platforms according to dose with natural severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection-induced antibody responses are limited.
Methods
Blood samples from adult patients with mild and severe COVID-19 and healthcare workers who received ChAdOx1 nCoV-19 vaccine (2nd dose at 12-week intervals) and BNT162b2 vaccine (2nd dose at 3-week intervals) were collected and compared by immunoglobulin G immune responses to SARS-CoV-2 specific spike protein using an in-house-developed enzyme-linked immunosorbent assay.
Results
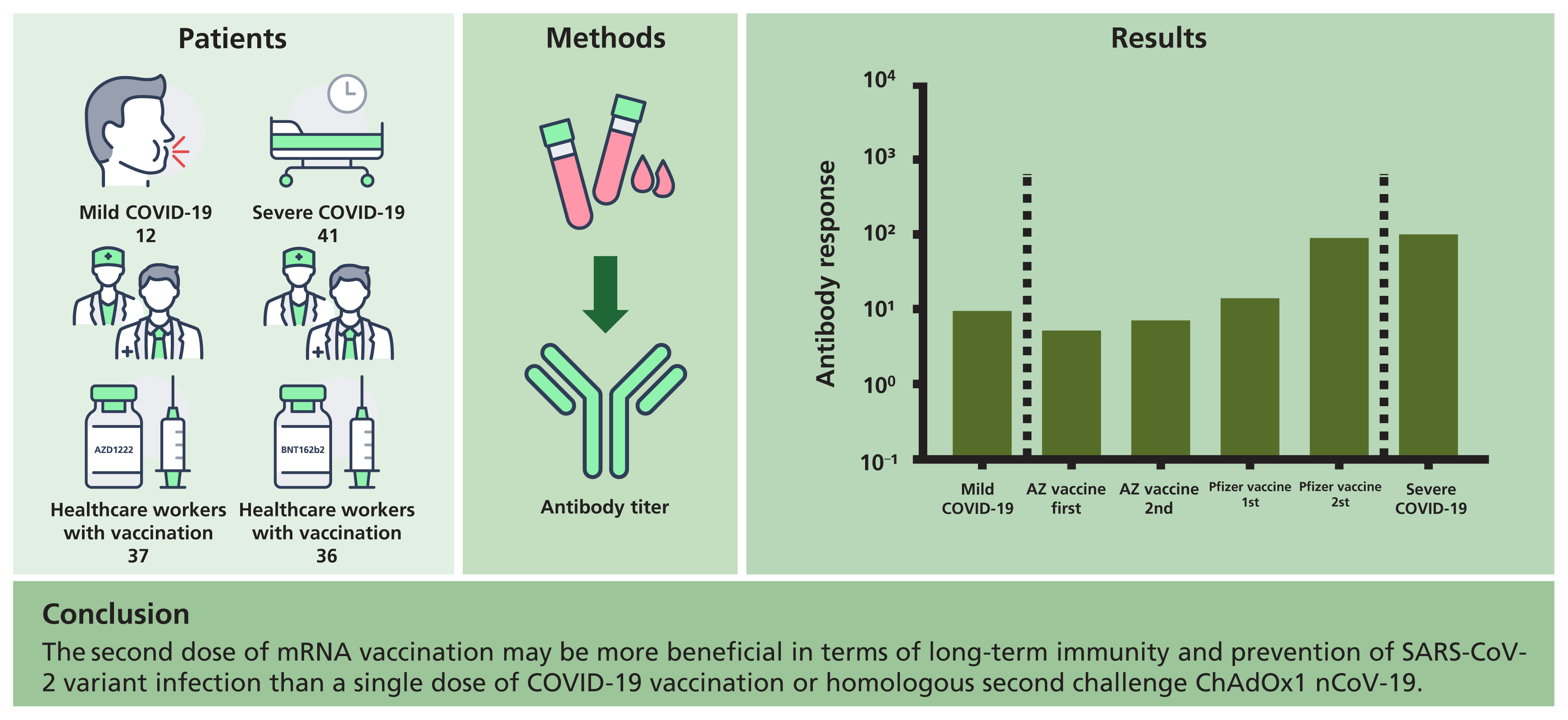
A total of 53 patients, including 12 and 41 with mild and severe COVID-19, respectively, were analyzed. In addition, a total of 73 healthcare workers, including 37 who received ChAdOx1 nCoV-19 and 36 who received BNT162b2, were enrolled. Antibody responses after the first and second doses of the ChAdOx1 nCoV-19 vaccine or the first dose of the BNT162b2 vaccine were similar to those in convalescent patients with mild COVID-19, but lower than those in convalescent patients with severe COVID-19, respectively. However, after the second dose of the BNT162b2 vaccine, the antibody response was comparable to that in convalescent patients with severe COVID-19.
As of September 2021, more than 6 billion people worldwide have received various platform coronavirus disease 2019 (COVID-19) vaccines with multiple dose regimens. However, vaccine shortages, the logistic speed of vaccination, the heterologous vaccination policy, and the concern of serious adverse effects of adenovirus-vector vaccines such as thrombosis with thrombocytopenia syndrome have left many people who have only been partially vaccinated. However, data comparing the antibody responses of these COVID-19 vaccine platforms according to dose are limited. In addition, comparing vaccine-induced immune responses with those in natural infection may provide further insight into the difference between vaccine- and natural infection-induced immunity. We thus compared antibody responses after the first and second doses of the ChAdOx1 nCoV-19 (ChAdOx1) and BNT162b2 vaccines with those in convalescent patients recovering from mild or severe COVID-19.
All COVID-19 infected patients admitted to Asan Medical Center, Seoul, South Korea, who agreed to blood sampling were prospectively enrolled between March 2020 and April 2021. The collected blood samples of patients with COVID-19 were analyzed at the convalescent phase (mean days from diagnosis ± standard deviation [SD], 29 ± 12). Disease severity was determined according to the National Institutes of Health (NIH) guidelines [1], and patients classified with mild or severe COVID-19 were finally assessed. In addition, healthcare workers (HCWs) who received the ChAdOx1 nCoV-19 vaccine or the BNT162b2 vaccine at Asan Medical Center between March 2021 and June 2021 and agreed with blood sampling were prospectively enrolled. Per the policies established by the Korean government, the BNT162b2 vaccine was assigned to high-risk HCWs in direct contact with patients with COVID-19, and the ChAdOx1 nCoV-19 vaccine was assigned to those working in general patient care. The blood samples of vaccinated HCWs were collected 3 weeks after the first dose of ChAdOx1 nCoV-19 or BNT162b2 and 2 weeks after the second dose of ChAdOx1 nCoV-19 or BNT162b2 vaccine. The interval between ChAdOx1 nCoV-19 and BNT162b2 doses were 12 and 3 weeks, respectively. This study was reviewed and approved by the Institutional Review Board of Asan Medical Center (IRB No. 2020-0297 and 2021-0170) and written informed consent was obtained from all participants..
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) plasma isolation in blood samples of patients with COVID-19 are described previously [2]. Human SARS-CoV-2 immunoglobulin G (IgG) antibodies were measured using an in-house-developed enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well plates (MaxiSorp, Thermo Fisher Scientific, Waltham, MA, USA) were coated with SARS-CoV-2 S1-His protein (2 μg/mL, SinoBiological, Beijing, China) overnight at 4°C, and then blocked with 1% bovine serum albumin in phosphate-buffered saline. Plasma was diluted (1:100, 1:1,000, or 1:10,000 for IgG) and incubated for 2 hours at room temperature. Horseradish peroxidase-conjugated anti-human IgG (Jackson Immunoresearch, West Grove, PA, USA) was used as secondary antibody. The plates were then developed with TMB substrate (Sigma-Aldrich, St. Louis, MO, USA), and the reaction was terminated using a stop solution (Sigma-Aldrich). Optical density (OD) was measured using a SpectraMax microplate reader (Molecular Devices LCC, San Jose, CA, USA) at 450 nm. Data are shown as relative OD values based on a 1:100 dilution factor.
To determine the cut-off values for the ELISA, we measured the mean values and SD of OD values from 12 negative control plasma that had not been exposed to SARS-CoV-2. The cut-off values were determined by calculating the mean OD plus three folds of the SD values, which was 0.4 for IgG as reported in previous studies [2].
Categorical variables were compared using the chi-square or Fisher’s exact test, and continuous variables using Student’s t test or the Mann-Whitney U test, as appropriate. According to the normality of the data, paired samples such as antibody responses after the first and second doses of the COVID-19 vaccines were compared using the paired t test or Wilcoxon rank-sum test. All tests of significance were two-tailed; p values of ≤ 0.05 were considered significant. The data were analyzed using SPSS version 24.0 (IBM Corp., Armonk, NY, USA), and graph plotting was performed using GraphPad Prism version 9 (GraphPad Software, San Diego, CA, USA).
A total of 53 patients, including 12 and 41 with mild and severe COVID-19, respectively, were analyzed. The baseline clinical characteristics of these patients are shown in Supplementary Table 1. In addition, a total of 73 healthcare workers, including 37 who received ChAdOx1 nCoV-19 vaccine and 36 who received BNT162b2 vaccine, were enrolled. No patients had previously been infected with SARS-CoV-2. The baseline characteristics of these healthcare workers are shown in Supplementary Table 1.
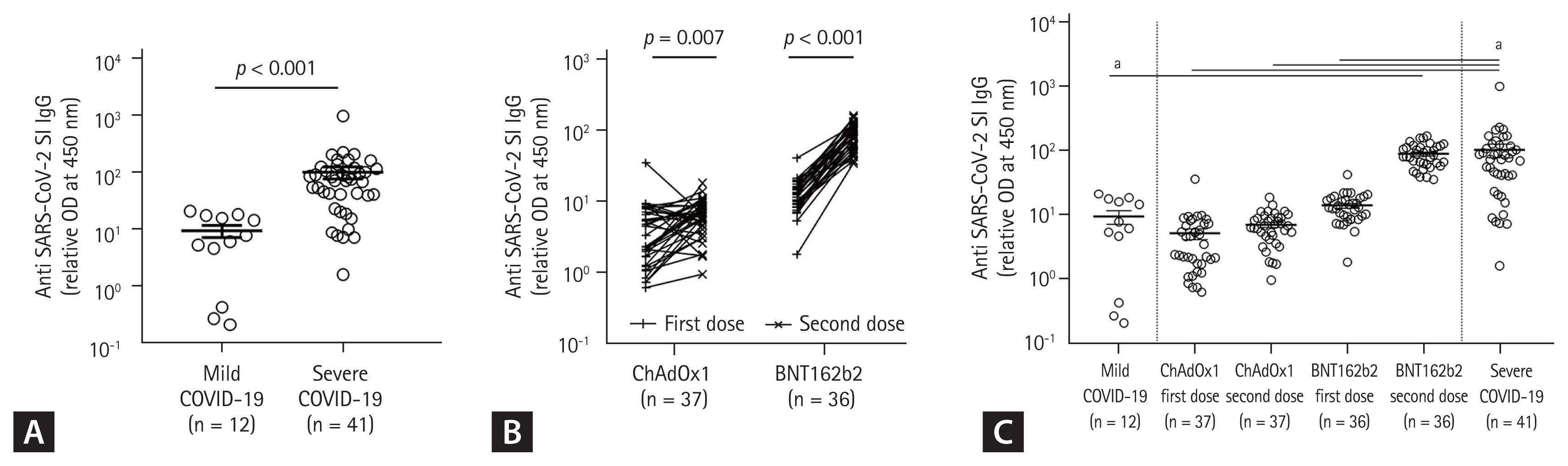
The SARS-CoV-2-specific IgG (S1-IgG) responses were significantly higher in convalescent patients with severe COVID-19 than in those with mild COVID-19 (mean levels ± SD 103.1 ± 157.7 and 9.44 ± 7.78, p < 0.001) (Fig. 1A). The antibody responses of the second dose of the ChAdOx1 nCoV-19 and BNT162b2 vaccinations were significantly higher than those after the first doses (p = 0.007 and p < 0.001, respectively) (Fig. 1B). Antibody responses after the first (5.14 ± 6.08) and second doses (7.03 ± 3.77) of the ChAdOx1 nCoV-19 vaccine, or the first dose (S1-IgG 14.03 ± 7.20) of the BNT162b2 vaccine, were similar to those in convalescent patients with mild COVID-19 (9.44 ± 7.78) but lower than those in convalescent patients with severe COVID-19 (103.1 ± 157.7) (Fig. 1C), respectively. However, the antibody response after the second dose (89.63 ± 35.98) of the BNT162b2 vaccine was similar to that in convalescent patients with severe COVID-19 (103.1 ± 157.7) (Fig. 1C).
Previous studies have consistently revealed that antibody responses were correlated with symptom severity in patients with COVID-19 [2,3]. In this study, we found that the second dose of BNT162b2 vaccine elicited a strong antibody response similar to that in patients who had recovered from severe COVID-19; however, the first dose of BNT162b2 or ChAdOx1 nCoV-19 and the second dose of ChAdOx1 nCoV-19 vaccination induced a weak antibody response, similar to that observed in patients who had recovered from mild COVID-19. In this contexture, the second dose of mRNA vaccination may be more beneficial in terms of long-term immunity and prevention of SARS-CoV-2 variant infection than a single dose of COVID-19 vaccination or homologous second challenge ChAdOx1 nCoV-19.
This study has some limitations. First, adenovirus-vector vaccine might elicite the peak antibody response later than mRNA vaccine, so the comparison of antibody response at the same time point between two vaccines may underestimate the antibody response by ChAdOx1 nCoV-19. Second, relatively small sample size in this study may be a limitation of further analysis. Also, the distributions of age were significantly different between groups due to the old age of severe COVID-19 infection group. Third, some may argue that in-house ELISA was not fully validated. However, the correlation between our in-house ELISA and commercial ELISA such as EUROIMMUN anti-SARS-CoV-2 ELISA (Euroimmun US Inc., Mountain Lakes, NJ, USA) was very strong (Supplementary Fig. 1). Finally, it is difficult to draw a firm conclusion for a protective immune response because data on functional antibody responses (such as neutralizing assays) are lacking. However, the S1-IgG used in this study had a moderate correlation with the neutralizing antibody assay using authentic SARS-CoV-2 (Supplementary Fig. 2). Furthermore, since the delta variant has been dominant in South Korea during writing this manuscript, we cannot draw any conclusion that a certain level of S1-IgG may be beneficial to protect the delta variant infection. In addition, the antigenic breadth covered by human antibody responses between vaccination and natural infection may vary [4,5], so cautious interpretation of our findings is required.
Despite these limitations, our data provide helpful information on the dose-dependent antibody responses of different COVID-19 vaccine platforms compared with those elicited by natural SARS-CoV-2 infection.
1. The antibody response after the second dose BNT162b2 vaccine was strongly induced compared to the first, second dose of ChAdOx1 nCoV-19 vaccine or the first dose of BNT162b2 vaccine.
2. Antibody responses after the first dose and second doses of ChAdOx1 nCoV-19 vaccine or the first dose of BNT162b2 vaccine were similar to that in convalescent patients with mild coronavirus disease 2019 (COVID-19), respectively, while that after the second dose of BNT162b2 vaccine was similar to that in convalescent patients with severe COVID-19.
Acknowledgments
This study was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) supported by the Korean Ministry of Health and Welfare, South Korea (grant No. HW20C2062).
Figure 1
Antibody responses after coronavirus disease 2019 (COVID-19) infection compared with COVID-19 vaccination. (A) Mild and severe COVID-19 infection. (B) First and second doses of ChAdOx1 or BNT161b2 vaccine. (C) COVID-19 natural infection and vaccination. SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; IgG, immunoglobulin G; OD, optical density. ap < 0.001.
REFERENCES
1. National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines [Internet] Bethesda (MD): National Institutes of Health, 2021. [cited 2022 Jan 25]. Available from: https://www.covid19treatmentguidelines.nih.gov
.
2. Kim JY, Kwon JS, Bae S, et al. SARS-CoV-2-specific antibody and T cell response kinetics according to symptom severity. Am J Trop Med Hyg 2021;105:395–400.



3. Lynch KL, Whitman JD, Lacanienta NP, et al. Magnitude and kinetics of anti-severe acute respiratory syndrome coronavirus 2 antibody responses and their relationship to disease severity. Clin Infect Dis 2021;72:301–308.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print



