 |
 |
| Korean J Intern Med > Volume 37(4); 2022 > Article |
|
Abstract
Background/Aims
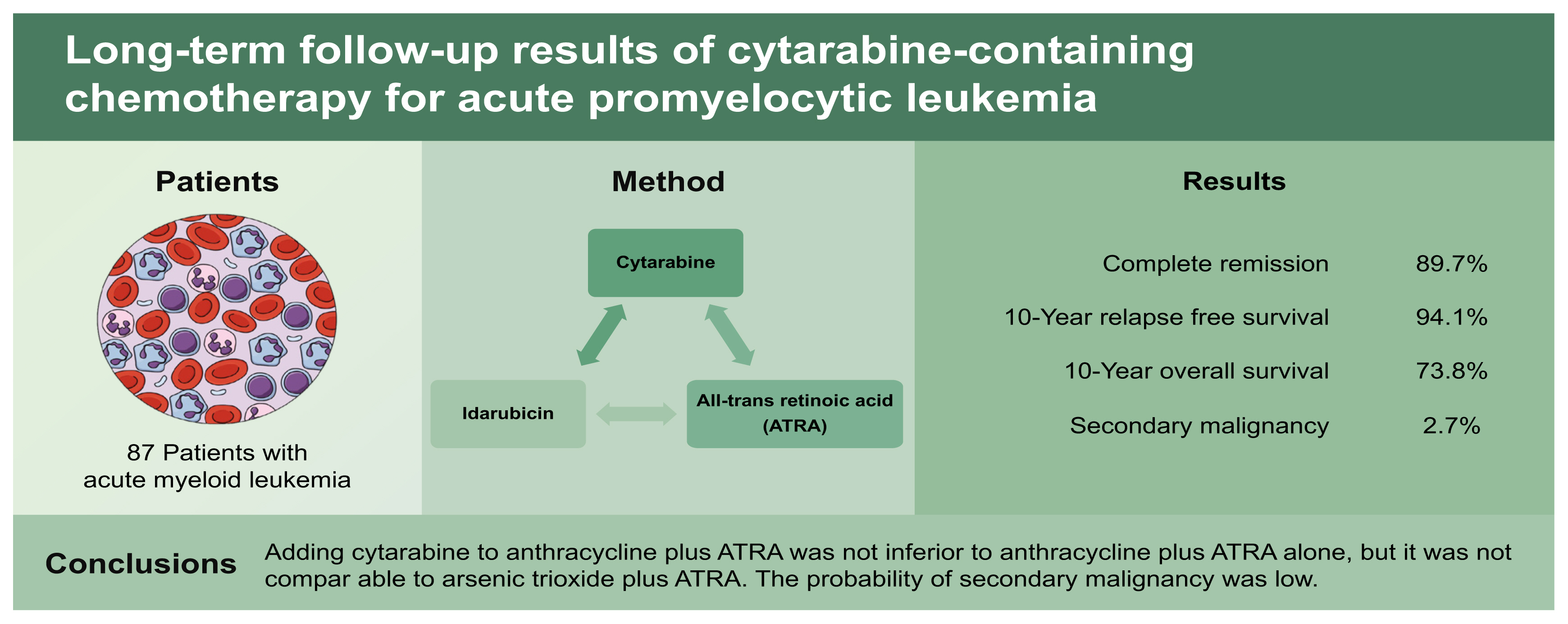
We evaluated the feasibility and long-term efficacy of the combination of cytarabine, idarubicin, and all-trans retinoic acid (ATRA) for treating patients with newly diagnosed acute promyelocytic leukemia (APL).
Methods
We included 87 patients with newly diagnosed acute myeloid leukemia and a t(15;17) or promyelocytic leukemia/retinoic acid receptor alpha (PML-RARα) mutation. Patients received 12 mg/m2/day idarubicin intravenously for 3 days and 100 mg/m2/day cytarabine for 7 days, plus 45 mg/m2/day ATRA. Clinical outcomes included complete remission (CR), relapse-free survival (RFS), overall survival (OS), and the secondary malignancy incidence during a 20-year follow-up.
Results
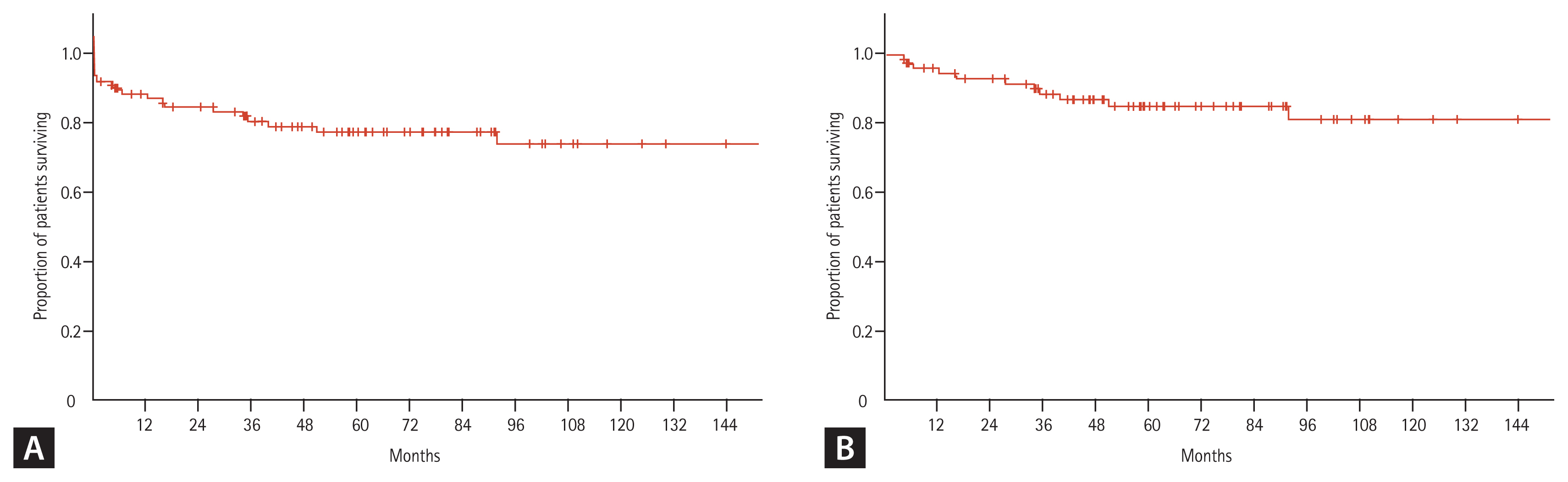
The CR, 10-year RFS, and 10-year OS rates were 89.7%, 94.1%, and 73.8%, respectively, for all patients. The 10-year OS rate was 100% for patients that achieved CR. Subjects were classified according to the white blood cell (WBC) count in peripheral blood at diagnosis (low-risk, WBC < 10,000/mm3; high-risk, WBC ≥ 10,000/mm3). The low-risk group had significantly higher RFS and OS rates than the high-risk group, but the outcomes were not superior to the current standard treatment (arsenic trioxide plus ATRA). Toxicities were similar to those observed with anthracycline plus ATRA, and higher than those observed with arsenic trioxide plus ATRA. The secondary malignancy incidence after APL treatment was 2.7%, among the 75 patients that achieved CR, and 5.0% among the 40 patients that survived more than 5 years after the APL diagnosis.
Acute promyelocytic leukemia (APL) is a distinct subtype of acute myeloid leukemia (AML) associated with t(15;17) and promyelocytic leukemia/retinoic acid receptor alpha (PML-RARα) gene mutations [1,2]. The prognosis of APL has been considered good, and thus, to date, it has been treated with a strategy different from that used for AML. The standard APL treatment is a combination of all-trans retinoic acid (ATRA) [3,4] with cytotoxic agents. The long-term prognosis of APL is favorable, except that a proportion of newly diagnosed patients may experience treatment-related morbidity and mortality. These outcomes are mainly due to bleeding related to secondary fibrinolysis and bleeding in the early phase of the treatment [5–7].
Recent advances in the treatment of APL have led to the combination of arsenic trioxide and ATRA. This combination produced a more favorable outcome than the classical combination of ATRA plus cytotoxic agents [8–10]. Nevertheless, the classical combination is widely used for newly diagnosed APL, due to issues with drug availability and cost-effectiveness.
Numerous previous studies have shown that the classical AML treatment, the combination of cytarabine and anthracycline, did not improve APL outcomes, compared to the single agent treatment with anthracycline. However, before the era of rapid testing for the PML-RARα mutation, chromosomal analysis was an essential in diagnosing APL, but it was a time-consuming process. This process introduced a critical delay in the diagnosis of APL. Moreover, rapid reduction of peripheral blasts is important in preventing life-threatening complications, like bleeding and differentiation syndrome, in the early phase of APL treatment. Thus, we reasoned that, before the PML-RARα mutation could be detected, it might be advantageous to administer cytarabine plus anthracycline, when AML is suspected, for rapid cytoreduction and the prevention of fatal complications.
In the present study, we evaluated the feasibility and efficacy of combining cytarabine, idarubicin, and ATRA for treating patients newly diagnosed with APL. The primary outcomes were long-term survival and the incidence of secondary malignancies among survivors during a 20-year follow-up.
This study was planned as one of the subgroup analyses of the AML-2000 protocol, which had been proposed as a risk-stratified treatment scheme, based on chromosome abnormalities in patients with newly diagnosed AML. The present analysis included patients with newly diagnosed AML that carried the t(15;17) or PML-RARα mutation (Fig. 1). The Institutional Review Boards of all participating institutions approved this study, and the approval number of the institute ‘Ewha Womans University Mokdong Hospital’ of the first author was EUMC 2016-09-014. Written informed consent by the patients was waived due to a retrospective nature of our study.
After the diagnosis, patients received 12 mg/m2/day idarubicin intravenously for 3 days and 100 mg/m2/day cytarabine for 7 days. ATRA was administered as an oral dose of 45 mg/m2/day after confirming the presence of Auer rods in the blood cell morphology, disseminated intravascular coagulation, and/or a t(15;17) or PML-RARα mutation. ATRA was continually administered until complete remission (CR) was confirmed.
At 28 days after the initiation of induction chemotherapy, when the absolute neutrophil and platelet counts recovered in peripheral blood (PB), a bone marrow biopsy was performed. CR was confirmed when the bone marrow blast count was < 5%, the absolute PB neutrophil count was > 1,000/mm3, and the platelet count was > 100,000/mm3. Patients that achieved CR received three sessions of consolidation treatment with 12 mg/m2/day idarubicin for 3 days without ATRA. ATRA was administered as maintenance therapy at an oral dose of 45 mg/m2/day for 15 days, every 2 months, for 2 years. Patients that failed to achieve CR received re-induction therapy, at the discretion of investigators, and either continued chemotherapy or received autologous/allogeneic stem cell transplantation after achieving CR.
Patients at standard risk, based on the PB white blood cell (WBC) count (< 10,000/mm3), received 45 mg/m2/day of ATRA for 15 days (every 2 months), as maintenance treatment for 2 years. For patients at high risk (PB WBC count ≥ 10,000/mm3), we administered the same dose of ATRA for 15 days (every 3 months), combined with 90 mg/m2/day 6-mercaptopurine (every day), plus 15 mg/m2/week methotrexate, and 1 mg/week folic acid, for 2 years.
Blood transfusions were performed during induction and consolidation chemotherapy regimens to maintain the hemoglobin level above 8 g/dL and the platelet count above 20,000/mm3. Prophylactic and empirical antibacterial, antifungal, and antiviral agents were administered at the discretion of each investigator.
Non-relapse mortality was defined as a death without disease progression, including hematologic relapse. Relapse-free survival (RFS) times were measured from the date of CR to the date of non-relapse mortality or hematologic relapse. Overall survival (OS) was measured from the APL diagnosis to the date of the last follow-up or death. RFS and OS were estimated with the Kaplan-Meier method and Cox-regression method. All statistical analyses were performed with IBM SPSS version 26 (IBM Co., Armonk, NY, USA). All data were analyzed and finally approved by all the authors.
From January 2000 to July 2007, a total of 605 patients with newly diagnosed AML had been screened at 22 institutions for the AML-2000 study. Among those, 50 patients were dropped, due to protocol violation. Thus, 555 patients were enrolled in the final study. Among those, we included 87 patients with APL in the present study.
The median age of our patients was 40 years (range, 6 to 80), and 10 patients were under 20 years old. The biologic features and hemogram status at diagnosis are shown in Table 1. Nineteen patients (21.8%) with PB WBC counts ≥ 10,000/mm3 were classified as the high-risk group. Then, for an alternate analysis, all patients were classified into three groups, based on the Programa de Estudio y Tratamiento de las Hemopatías Malignas (PETHEMA) classification system, according to their WBC and PB platelet counts at diagnosis, as follows: high-risk, WBC ≥ 10,000/mm3; intermediate-risk, WBC < 10,000/mm3 and platelet < 40,000/mm3; low-risk, WBC < 10,000/mm3 and platelet ≥ 40,000/mm3 [11]. According to that system, 29, 31, and 16 patients were included in high-, intermediate-, and low-risk groups, respectively.
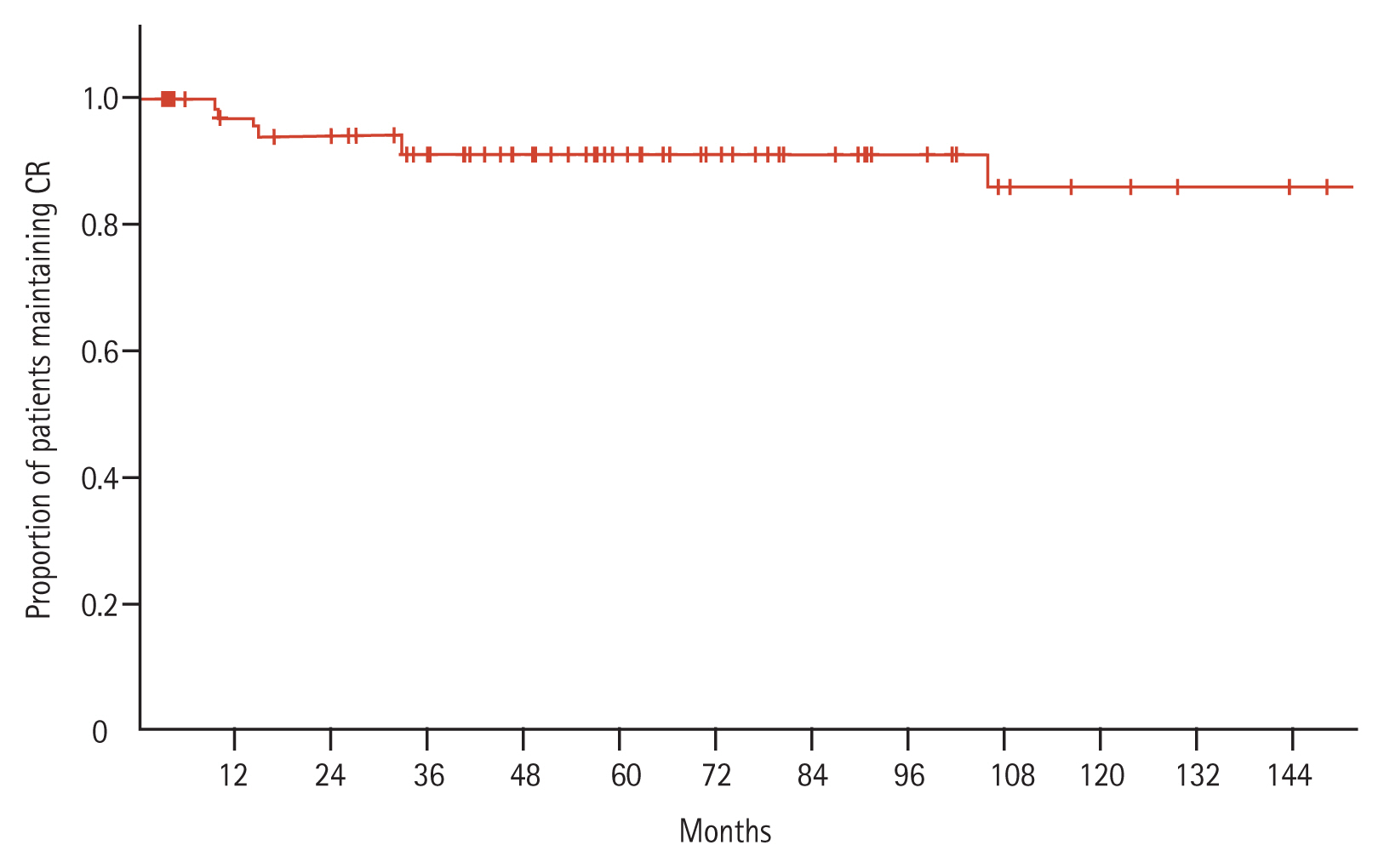
A total of 75 patients (86.2%) achieved CR after induction chemotherapy. An additional three patients achieved CR with additional chemotherapy. Accordingly, the CR rate was 89.7%. The median time from the initiation of induction chemotherapy to the achievement of CR was 29 days (range, 14 to 84). Among the patients that failed to achieve CR, seven failed due to treatment-related mortality (5, bleeding; 1, infection; 1, organ failure), and two had primary-refractory disease.
When all patients were classified into two groups, according to their initial WBC count (low-risk, < 10,000/mm3; high-risk, ≥ 10,000/mm3), the CR rate was higher in the low-risk group than in the high-risk group (87.9% vs. 78.9%), but the difference between groups was not significant. When patients were classified into three groups, according to their WBC and platelet counts, the CR rates tended to decrease with increasing risk (low-risk, 96.7%; intermediate-risk, 80.6%; high-risk, 78.9%; p = 0.059).
After a median follow-up period of 73.5 months, the median RFS was not reached, but the 10-year RFS rate was 94.1% (Fig. 2). Similarly, the median OS was not reached, but the 10-year OS rates were: 73.8% for all patients and 100% for patients that achieved CR (Fig. 3).
When all patients were classified into two groups, according to the PB WBC count at diagnosis (low-risk, WBC < 10,000/mm3; high-risk WBC ≥ 10,000/mm3), both the RFS and OS of patients in the low-risk group were significantly higher than those in the high-risk group (Fig. 4). When the patients were classified into three groups according to the PB WBC and platelet counts at diagnosis, the RFS and OS of patients in each risk group were significantly different. The 10-year RFS rates were: 90.0% in the low-risk group, 96.0% in the intermediate-risk group, and 73.3% in the high-risk group (p = 0.22). Similarly, the 10-year OS rates were 93.2%, 62.5%, and 57.9%, respectively (p = 0.006) (Fig. 5).
Table 2 shows all the toxicities and all the grade 3–4 toxicities observed during the induction and consolidation treatment periods. Although cytarabine and idarubicin were administered together during the induction period, the proportion of patients that experienced nausea/vomiting was only 62% (all grades), and only 5% were grades 3–4 severity. Most patients (80%) experienced neutropenic fever, and 39% of patients experienced grade 3–4 neutropenic fever during the induction period. Any kind of infection was observed in 37% of patients (all grades), and 29% had grade 3–4 infections. Only a modest proportion of adverse events occurred during the consolidation periods, and no patients died due to toxicity or dropped out due to adverse events.
When we defined early death during induction as death from any cause for up to 28 days of induction, the early death rate was 10.3%. The causes of death were bleeding (n = 5), multiorgan failure (n = 2), infection (n = 1), and refractory disease (n = 1).
Among the patients enrolled in this study, two patients experienced secondary malignancy after the APL treatment. Thus, the incidences of secondary malignancy after the APL treatment were 2.7%, among the 75 patients that achieved CR, and 5.0%, among the 40 patients that survived more than 5 years after the APL diagnosis. Of these two patients, one developed colon cancer at 71 months after the APL diagnosis, and the other developed esophageal cancer at 123 months after the APL diagnosis. These two patients survived after the secondary malignancy treatment without any evidence of APL relapse.
This study analyzed the treatment outcomes of patients with newly diagnosed APL that were treated according to the AML-2000 protocol. The AML-2000 protocol was initiated in January 2000 and was proposed as a risk-stratified treatment scheme, based on chromosome abnormalities, for newly diagnosed AML. However, numerous previous studies [3,4,11–16] have shown that, when the anthracycline plus cytarabine combination was administered as an induction chemotherapy, patients did not experience better outcomes compared to those treated with anthracycline alone. Currently, anthracycline plus ATRA is one of the standard treatment options for induction chemotherapy in patients with newly diagnosed APL. In addition, arsenic trioxide plus ATRA was recently recommended as a better treatment option for APL [2,8–10, 17–19].
At the time that we proposed the AML-2000 protocol, it was not fully known whether ATRA combined with cytarabine and anthracycline was superior to ATRA plus anthracycline for newly diagnosed APL. Thus, we intended to evaluate whether the three-drug combination regimen could overcome the poor outcome of high-risk APL. In 2000, rapid genetic tests for identifying the presence of PML-RARα were not widely used in clinical practice; thus, the definite diagnosis of APL was delayed until a chromosome analysis could confirm the presence of t(15;17). We reasoned that an upfront, rapid initiation of the AML-type induction chemotherapy (anthracycline and cytarabine) without delay for patients with suspected APL might rapidly eliminate the promyelocytes, suppress the progression of secondary fibrinolysis, and reduce early treatment-related morbidity/mortality, including life-threatening bleeding episodes [3,5–7,16,20,21].
Recently, studies have reported overall outcomes for patients with APL treated with ATRA or arsenic trioxide plus chemotherapy. In a randomized trial by the French Belgian Swiss (FBS) APL group [8], patients with APL were treated with ATRA plus idarubicin and cytarabine, followed by consolidation chemotherapy with idarubicin combined with cytarabine, arsenic trioxide, or ATRA. In the standard-risk group, the 5-year event-free survival rates were 88.7%, 95.7%, and 85.4%, among patients treated with idarubicin and cytarabine, arsenic trioxide, or ATRA, respectively. In the high-risk group, the 5-year event-free survival rates were 85.5% and 92.1%, among patients in the chemotherapy and chemotherapy plus arsenic trioxide groups, respectively.
In the present study, we first classified all patients according to the PB WBC count at diagnosis (low-risk, WBC < 10,000/mm3 vs. high-risk, WBC ≥ 10,000/mm3). We found that, for patients in the low-risk group, the 10-year RFS and OS rates were 90.9% and 80.0% respectively, which were significantly higher than those observed in the high-risk group (RFS 73.3% and OS 57.9%) (Fig. 4). These outcomes were somewhat inferior to the 5-year event-free survival rates of patients treated with idarubicin plus arsenic trioxide in the FBS study (standard risk 95.7%, high-risk 92.1%). However, the 10-year RFS of the low-risk group in this study (90.9%) was comparable to the 5-year RFS of patients treated with idarubicin plus cytarabine or ATRA in the low-risk group of the FBS study (88.7% and 85.4%, respectively). For high-risk patients, in this study, the 10-year RFS (73.3%) was somewhat inferior to the 5-year event-free survival rates of the FBS high-risk APL groups that received chemotherapy (85.5%) or chemotherapy plus arsenic trioxide (92.1%), even though ATRA was also combined with chemotherapy in the present study.
At the initiation of the present study, arsenic trioxide was not available. At that time, the treatment option for overcoming the adverse prognostic factors associated with highrisk APL was to add cytarabine to idarubicin. Therefore, we tested this combination. However, that treatment was not superior to idarubicin plus ATRA in the standard-risk group, and it was inferior to idarubicin plus arsenic trioxide in the high-risk group. Consequently, the idarubicin plus ATRA or arsenic trioxide combinations are currently recommended for treating APL, and arsenic trioxide plus ATRA is recommended for high-risk patients.
When the patients in this study were classified according to either the PB WBC count at diagnosis or the PB WBC and platelet counts at diagnosis, the RFS and OS were significantly different between groups. Those results suggested that the addition of cytarabine to anthracycline plus ATRA as an induction chemotherapy could overcome the poor outcome of high-risk APL. These findings were well correlated with recent recommendations and study outcomes for newly diagnosed APL.
In the present study, the treatment-related mortality rate was 8.0% during induction chemotherapy, which was lower than the rates reported in previous studies (27% to 47%) [22,23], even though we added cytarabine to the induction chemotherapeutic regimen. However, considering that arsenic trioxide plus ATRA is currently recommended as the standard therapy for APL, our overall adverse event rates, including infection and neutropenic fever, were relatively higher than the rates associated with the current standard therapy. Nevertheless, the idarubicin, cytarabine, plus ATRA combination can be considered in developing countries with special situations; for example, in some countries, the cost of arsenic trioxide plus ATRA is not reimbursed, and it is not well tolerated. In the past, when rapid tests for detecting PML-RARα were not available, the early administration of cytotoxic agents with a common regimen for AML might have been more beneficial than the delayed administration of an APL-specific regimen, after detecting the specific chromosomal abnormality for APL. In those cases, the rapid reduction of promyelocytes reduced the tendency for bleeding associated with secondary fibrinolysis in APL. However, recent advances in diagnostic tests for detecting genetic mutations related to AML can provide information rapidly, and APL can be diagnosed in a few days. Therefore, currently, APL-specific induction treatments can be initiated without delay. Accordingly, the early administration of AML-type induction chemotherapy, including cytarabine, is no longer recommended.
We conducted long-term follow-ups to monitor long-term survival and the development of secondary malignancy related to chemotherapy [10,18,24]. To our knowledge, this study was the first to report the development of secondary malignancy among survivors treated for APL, except for one report based on Surveilance, Epidemiology, and End Results Program (SEER)-Medicare data [25]. Among the 87 patients included in this study, 14 were followed for more than 10 years, and 40 patients were followed for at least 5 years and up to 20 years.
The main limitation of the current study was its retrospective design. In addition, the sample size was relatively small, which limited our ability to conclude definitively that the combination of anthracycline and cytarabine as an induction chemotherapy was comparable to the current standard anthracycline-based regimen for APL. Moreover, the long-term sample was too small to assure safety, in terms of the development of a secondary malignancy. Another limitation was that we started the induction therapy with the combination of idarubicin, cytarabine, plus ATRA without stratification according to patient risk features, because this study was initiated in the absence of the specific, powerful tool for detecting the presence of PML-RARα. Consequently, the results of this study should be interpreted and applied with caution. Finally, the current standard treatment for APL has been established as the combination of ATRA and arsenic trioxide or anthracycline, and those treatments are preferred to anthracycline plus cytarabine.
In conclusion, this study showed that the AML-type standard chemotherapeutic regimen of cytarabine combined with idarubicin plus ATRA did not improve treatment outcomes in patients with high-risk APL. The addition of cytarabine to anthracycline plus ATRA was not inferior to anthracycline plus ATRA alone, in terms of treatment-related morbidity and mortality, or in terms of treatment outcome. However, cytarabine added to anthracycline plus ATRA was inferior to arsenic trioxide plus ATRA. Our results suggested that cytarabine added to anthracycline plus ATRA could be used for treating patients with newly diagnosed APL, when rapid genetic tests are not available, when the early differential diagnosis of APL (i.e., distinguishing APL from other types of AML) is difficult, or when the standard arsenic trioxide plus ATRA treatment is unaffordable. Finally, this study showed that the probability of secondary malignancy was not high. Indeed, among patients with APL that achieved and maintained CR for more than 5 years after cytarabine, anthracycline, plus ATRA, most survived for more than 10 years.
Figure 1
Consort diagram. CR, complete remission; TRM, treatment-related mortality; BMT, bone marrow transplantation.

Figure 4
Survival curves, according to the risk group, based on the initial white blood cell (WBC) count. (A) Leukemia-free survival. (B) Overall survival (total patients). (C) Overall survival (among patients who achieved complete remission [CR]).
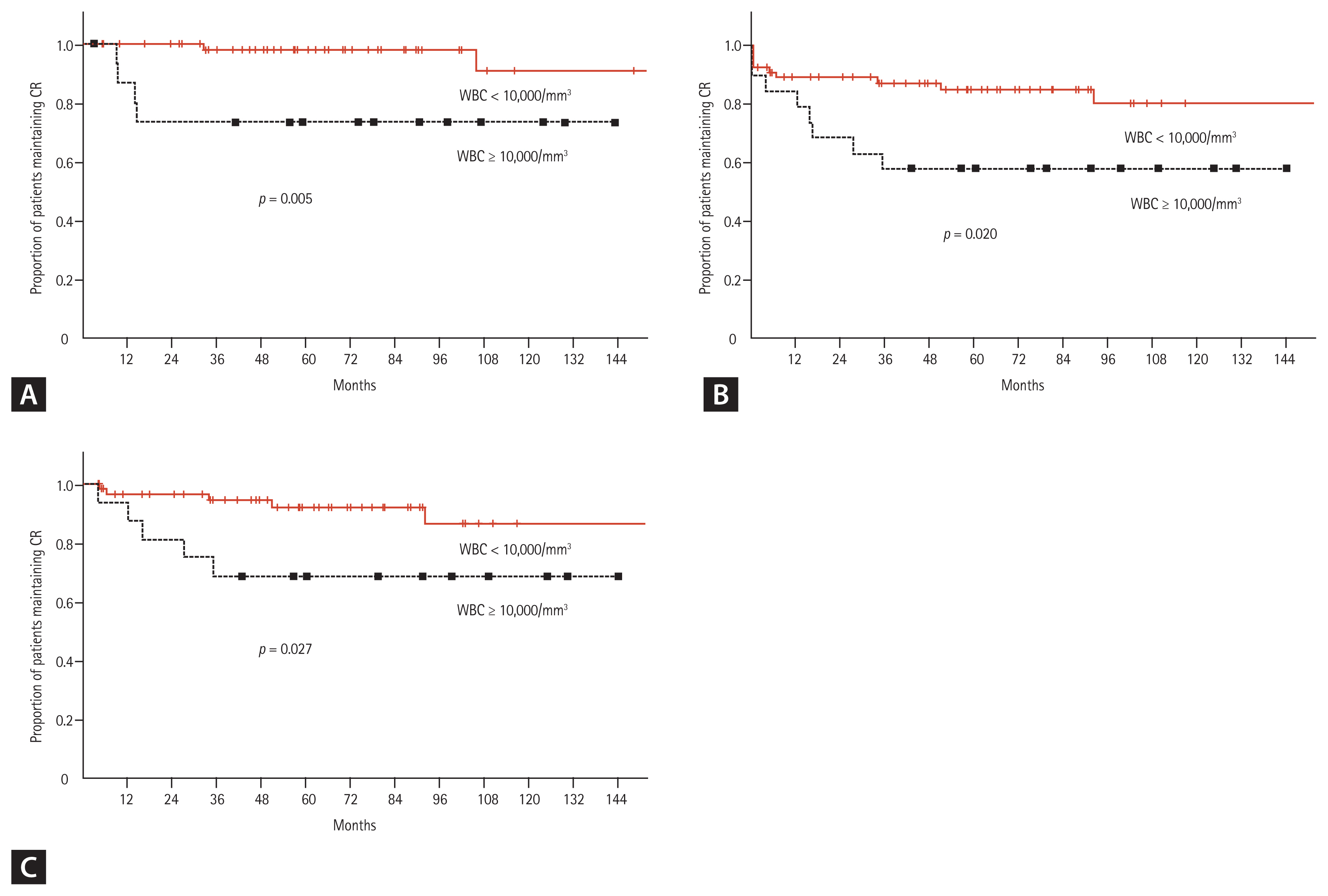
Figure 5
Survival curves, according to the risk group, based on the initial white blood cell (WBC) and platelet counts. (A) Leukemia-free survival. (B) Overall survival (total patients). CR, complete remission.
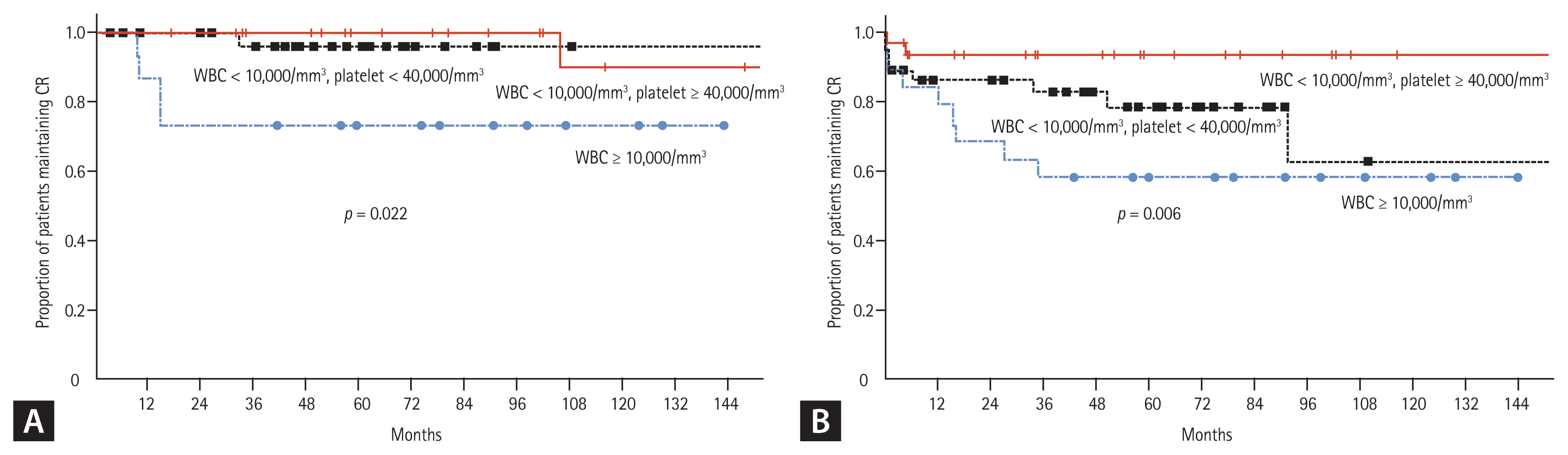
Table 1
Demographics of patients (n = 87)
Table 2
Adverse effects during each chemotherapeutic cycle
REFERENCES
2. Marty M, Ganem G, Fischer J, et al. Acute promyelocytic leukemia: retrospective study of 119 patients treated with daunorubicin. Nouv Rev Fr Hematol 1984;26:371–378.

3. Dombret H, Sutton L, Duarte M, et al. Combined therapy with all-trans-retinoic acid and high-dose chemotherapy in patients with hyperleukocytic acute promyelocytic leukemia and severe visceral hemorrhage. Leukemia 1992;6:1237–1242.

5. Krsnik I, Escriba A, Lopez-Rubio M, Alarcon C, del Potro E, Diaz-Mediavilla J. Bleeding in acute promyelocytic leukemia (APL): fibrinolysis or defibrination? Nouv Rev Fr Hematol 1991;33:39–41.

6. Sakata Y, Murakami T, Noro A, Mori K, Matsuda M. The specific activity of plasminogen activator inhibitor-1 in disseminated intravascular coagulation with acute promyelocytic leukemia. Blood 1991;77:1949–1957.



7. Stein E, McMahon B, Kwaan H, Altman JK, Frankfurt O, Tallman MS. The coagulopathy of acute promyelocytic leukaemia revisited. Best Pract Res Clin Haematol 2009;22:153–163.


8. Ades L, Thomas X, Bresler AG, et al. Arsenic trioxide is required in the treatment of newly diagnosed acute promyelocytic leukemia: analysis of a randomized trial (APL 2006) by the French Belgian Swiss APL group. Haematologica 2018;103:2033–2039.



9. Zhu HH, Hu J, Lo-Coco F, Jin J. The simpler, the better: oral arsenic for acute promyelocytic leukemia. Blood 2019;134:597–605.



10. Cicconi L, Platzbecker U, Avvisati G, et al. Long-term results of all-trans retinoic acid and arsenic trioxide in non-high-risk acute promyelocytic leukemia: update of the APL0406 Italian-German randomized trial. Leukemia 2020;34:914–918.



11. Sanz MA, Martin G, Gonzalez M, et al. Risk-adapted treatment of acute promyelocytic leukemia with all-trans-retinoic acid and anthracycline monochemotherapy: a multicenter study by the PETHEMA group. Blood 2004;103:1237–1243.


12. Avvisati G, Petti MC, Lo-Coco F, et al. Induction therapy with idarubicin alone significantly influences event-free survival duration in patients with newly diagnosed hypergranular acute promyelocytic leukemia: final results of the GIMEMA randomized study LAP 0389 with 7 years of minimal follow-up. Blood 2002;100:3141–3146.

13. Sanz MA, Grimwade D, Tallman MS, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood 2009;113:1875–1891.



14. Coombs CC, Tavakkoli M, Tallman MS. Acute promyelocytic leukemia: where did we start, where are we now, and the future. Blood Cancer J 2015;5:e304.




15. Cicconi L, Lo-Coco F. Current management of newly diagnosed acute promyelocytic leukemia. Ann Oncol 2016;27:1474–1481.


16. Li X, Wang C, Chen G, Ji B, Xu Y. Combined chemotherapy for acute promyelocytic leukemia: a meta-analysis. Hematology 2017;22:450–459.


17. Platzbecker U, Avvisati G, Cicconi L, et al. Improved outcomes with retinoic acid and arsenic trioxide compared with retinoic acid and chemotherapy in non-high-risk acute promyelocytic leukemia: final results of the randomized Italian-German APL0406 Trial. J Clin Oncol 2017;35:605–612.


18. Gill H, Yim R, Lee HKK, et al. Long-term outcome of relapsed acute promyelocytic leukemia treated with oral arsenic trioxide-based reinduction and maintenance regimens: a 15-year prospective study. Cancer 2018;124:2316–2326.



19. Sanz MA, Fenaux P, Tallman MS, et al. Management of acute promyelocytic leukemia: updated recommendations from an expert panel of the European LeukemiaNet. Blood 2019;133:1630–1643.




20. Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood 2008;111:2505–2515.



21. Eghtedar A, Rodriguez I, Kantarjian H, et al. Incidence of secondary neoplasms in patients with acute promyelocytic leukemia treated with all-trans retinoic acid plus chemotherapy or with all-trans retinoic acid plus arsenic trioxide. Leuk Lymphoma 2015;56:1342–1345.



22. Cordonnier C, Vernant JP, Brun B, et al. Acute promyelocytic leukemia in 57 previously untreated patients. Cancer 1985;55:18–25.


23. Hoyle CF, Swirsky DM, Freedman L, Hayhoe FG. Beneficial effect of heparin in the management of patients with APL. Br J Haematol 1988;68:283–289.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



