 |
 |
| Korean J Intern Med > Volume 37(5); 2022 > Article |
|
Abstract
Background/Aims
The optimal systolic blood pressure (SBP) goal for elderly patients with hypertension, especially to reduce cardiovascular disease (CVD) incidence and improve outcome, is unclear. This study aimed to evaluate the beneficial effects of intensive treatment for hypertension on the incidence of CVD in elderly Korean patients.
Methods
The HOW to Optimize eLDerly systolic Blood Pressure (HOWOLD-BP) trial is a multicenter, parallel-design, open-label, randomized controlled trial designed to evaluate whether intensive treatment (SBP Ōēż 130 mmHg) will provide more benefits in lowering the incidence and mortality associated with CVD than standard treatment (SBP Ōēż 140 mmHg) in elderly patients with hypertension aged Ōēź 65 years. For this study, eleven university hospitals in Korea will enroll approximately 3,176 elderly patients with hypertension between 2019 and 2022. Patients will be requested to visit the clinic every 4 months for the first year and every 6 months thereafter for 36 months. Parameters, including clinic and home blood pressure, anthropometric and laboratory findings, and frailty assessments, will be collected according to the standardized protocol. The primary outcome is a composite of CVD (acute coronary syndrome, stroke, and heart failure) incidence and cardiovascular deaths.
Hypertension has become a significant global health problem owing to its high prevalence and associated complications, such as cardiovascular disease (CVD) [1,2]. The prevalence of hypertension also increases with age. According to data from the National Health and Nutrition Examination Survey, the prevalence of hypertension among United States adults from 2017 to 2018 was 22.4% among those aged 18 to 39 years, 54.5% among those aged 40 to 59 years, and 74.5% among those aged Ōēź 60 years [3]. A similar trend was observed in Korea according to the Korea Hypertension Fact Sheet 2020, which showed an increasing prevalence of hypertension with age for both men and women [4].
In many previous studies, aggressive antihypertensive therapy has been shown to reduce the incidence of CVD [5]. However, the optimal systolic blood pressure (SBP) target for elderly patients remains uncertain. Several large-scale trials have been performed to evaluate the effect of intensive blood pressure (BP) control on the incidence of CVD in elderly hypertensive patients [6ŌĆō13]. However, these studies have reported conflicting results. Therefore, the optimal target BP for elderly patients with hypertension differs according to each countryŌĆÖs hypertension guidelines. The target SBP for elderly patients with hypertension is < 130 mmHg according to the 2017 American College of Cardiology/American Heart Association guidelines, between 130 and 139 mmHg in the 2018 European Society of Cardiology/European Society of Hypertension guidelines, and < 140 mmHg in the 2018 Korean Society of Hypertension (KSH) guidelines [14ŌĆō16].
For elderly patients, several characteristics of hypertension and other special considerations associated with aging must be taken into account. For example, as a consequence of increased arterial stiffness that occurs throughout the aging process, the pulse wave velocity increases, and the reflected waves return faster. Therefore, an increase in SBP and pulse pressure has been observed in elderly patients with hypertension [17]. Patients also frequently experience several adverse events during treatment, including orthostatic hypotension, dizziness, and falls. Moreover, with elderly patients, frailty, multiple comorbidities, polypharmacy, cognitive impairment, and depression may co-exist. These situations must be considered when treating elderly patients with hypertension.
We are conducting the HOW to Optimize eLDerly systolic Blood Pressure (HOWOLD-BP) trial to compare the incidence of cardiovascular (CV) events in elderly Korean patients with hypertension based on two target SBP levels (Ōēż 130 and Ōēż 140 mmHg). We will also evaluate whether frailty status, assessed using the frailty questionnaire, gait speed, and handgrip strength, modifies the effect of the BP control strategy. Additionally, home BP data will be collected and analyzed along with the clinic BP profile.
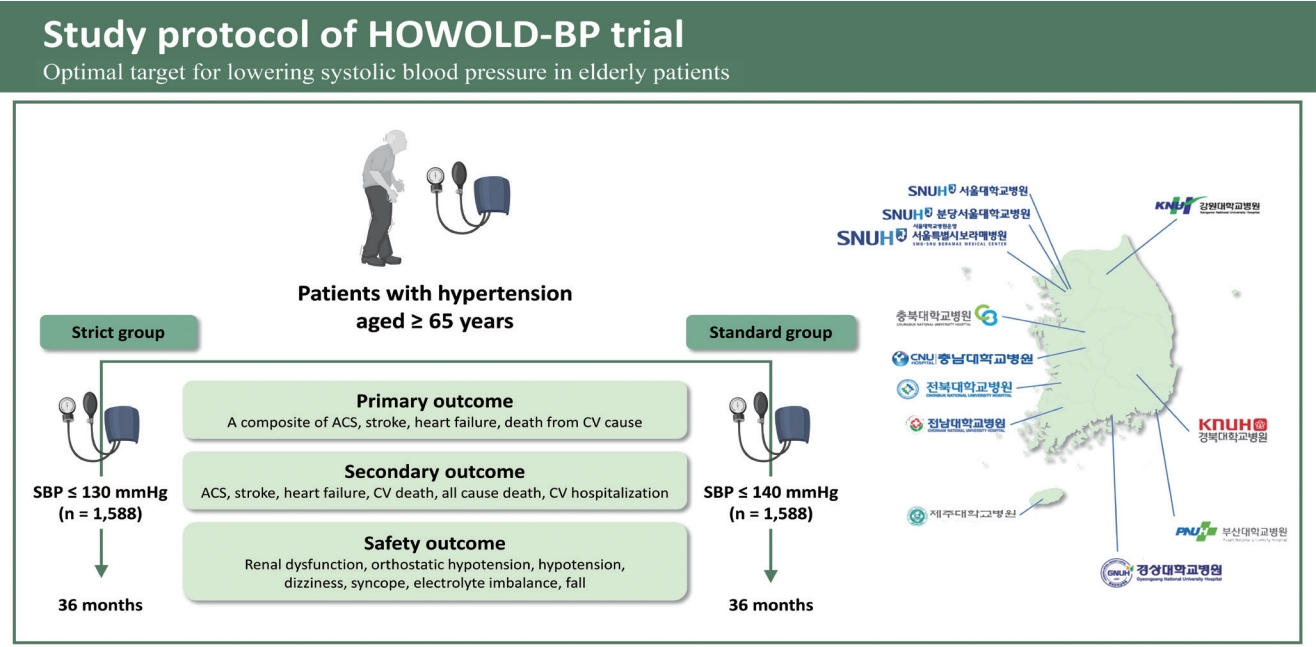
The HOWOLD-BP trial is designed as a prospective, multicenter, open-label randomized clinical trial and patient follow-up is planned for 3 years. The primary purpose of the trial is to evaluate whether intensive SBP-lowering treatment (target SBP Ōēż 130 mmHg) reduces CV events compared with standard treatment (target SBP Ōēż 140 mmHg) in Korean patients with hypertension aged Ōēź 65 years.
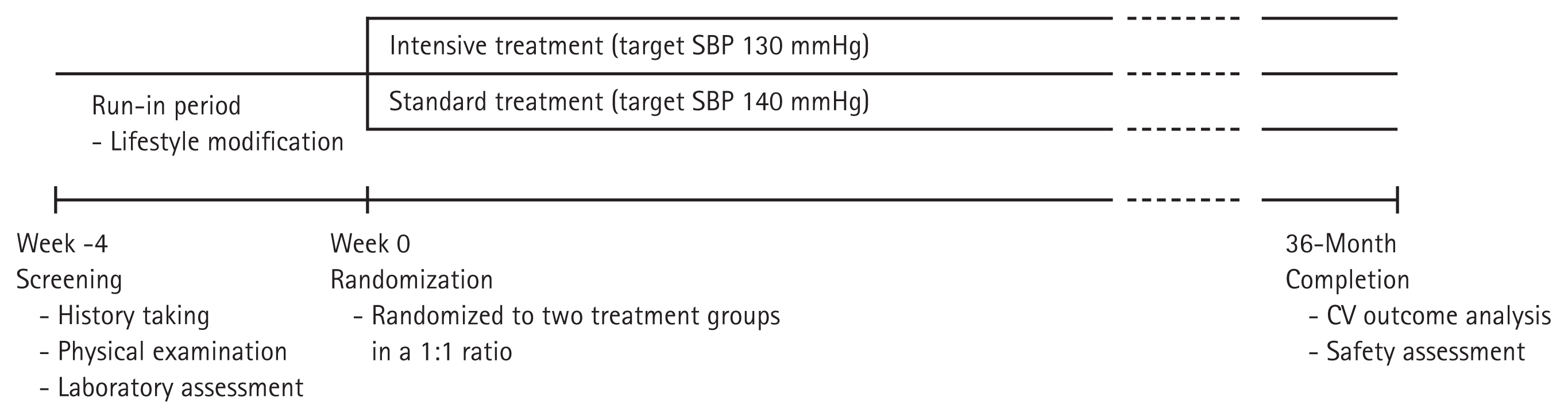
Subjects will be randomized into two groups according to the SBP target: Ōēż 130 mmHg (intensive treatment group) and Ōēż 140 mmHg (standard treatment group). The subjects have been recruited from 11 university hospitals in Korea since January 2019. Subjects will be enrolled for 4 years, and each individual is planned to be followed up for 3 years (Fig. 1). During the follow-up period, patients will be requested to visit the hospital at least seven times, and information regarding clinic and home BP, supine and standing BP, laboratory findings, frailty assessments, and drug compliance will be collected, and a questionnaire for quality of life (QOL) and cognitive function will be performed according to the standardized protocol (Table 1).
The study protocol has been approved by the ethics committee of each participating center (The Institutional Review Board at Chungbuk National University Hospital, approval No. 2018-09-015). This study was registered with the Clinical Research Information Service (Internet), Osong (Chungcheongbuk-do): Korea Centers for Disease Control and Prevention, Ministry of Health and Welfare (Republic of Korea) in 2019 (KCT0003787, available from: https://cris.nih.go.kr/).
The inclusion and exclusion criteria for enrolment are shown in Table 2. We will include patients aged Ōēź 65 years with hypertension who were already taking antihypertensive medications or treatment-na├»ve patients with a clinic SBP of 140 to 180 mmHg. All patients enrolled in this study must be independent in their activities of daily living (ADL). Patients will be excluded from the study if they have secondary hypertension, resistant hypertension (BP Ōēź 140/90 mmHg with three classes of antihypertensive medications or maintenance of BP < 140/90 mmHg with more than four different classes of antihypertensive medications, including diuretics), or orthostatic hypotension (1-minute standing SBP < 110 mmHg) with symptoms at screening. Patients with a history of acute coronary syndrome (ACS), cardiac surgery, urgent coronary intervention, or stroke within 3 months and patients with concomitant systolic heart failure or other significant structural heart diseases will also be excluded. Patients with uncontrolled diabetes mellitus (hemoglobin A1c [HbA1c] Ōēź 10%), end-stage renal disease (on hemodialysis or estimated glomerular filtration rate [eGFR] less than 15 mL/min/1.73 m2), severe liver function abnormality (liver enzyme levels greater than three times the upper normal range), uncontrolled thyroid dysfunction, and moderate to severe retinopathy will also be excluded.
Eligible patients will be screened by a local investigator based on the inclusion and exclusion criteria. During the run-in period, patientsŌĆÖ suitability for the study will be assessed according to the criteria. Eligible patients will be requested to continue their previous medication regimens and lifestyle modifications. Within 4 weeks of screening, eligible patients will be randomly allocated at a ratio of 1:1 to one of the two groups according to the target SBP (Ōēż 130 mmHg, intensive treatment group or Ōēż 140 mmHg, standard treatment group). The clinical sites at randomization are considered stratification factors for randomization. All patients participating in the study will be informed of the study and sign written consent.
After randomization, we plan to follow-up each patient for 3 years. During the follow-up period, patients will be requested to visit the hospital every 4 months in the first year and every 6 months in the second and third years (i.e., at month 1, 4, 8, 12, 18, 24, 30, and 36) (Table 1). Patient information, such as demographics, comorbidities, laboratory findings, cognitive function, and assessment of QOL and frailty, will be collected per protocol. Medication compliance, calculated as [number of taken pills/number of prescribed pills] ├Ś 100, will also be evaluated at every visit after randomization.
At the initial visit (Visit 1, at the time of screening), demographic information will be collected, including the participantsŌĆÖ initials, sex, date of birth, age, and registration number. Each participant will undergo a comprehensive physical examination, medical history, frailty assessment, cognitive function test, and QOL questionnaire. Trained clinical research coordinators will administer the interviews and questionnaires. Clinical data will include current and past medical history and medications, especially for hypertension, diabetes mellitus, dyslipidemia, and anticoagulants.
BP and pulse rate will be measured at the heart level in the seated position using a professional digital BP monitor (HEM7080-IC, Omron Healthcare, Lake Forest, IL, USA). The measurements will be performed in a quiet room after 5 minutes of rest and repeated three times after another 2 minutes of rest under the supervision of the research coordinator. Assessment of orthostatic hypotension will be conducted at enrollment and at every clinic visit during follow-up. Orthostatic hypotension is defined as a decrease in SBP of at least 20 mmHg or a decrease in diastolic blood pressure (DBP) of at least 10 mmHg within 3 minutes of standing [18]. During the follow-up period, all patients will be requested to measure and record their BP while performing daily activities at home and bring it when they visit the hospital.
Trained clinical research coordinators will assess frailty. ADLs will be evaluated using the modified Barthel index (range, 0 to 100; lower numbers indicate worse frailty) [19]. Instrumental ADLs (IADLs) will be measured using the Lawton and Brody index (range, 0ŌĆō5 for males; 0ŌĆō8 for females; lower numbers indicate worse IADL function) [20]. ADLs range from 0 to 100, with 100 indicating full independence, 75 to 99 indicating partial dependence, and < 75 indicating full dependence. For IADLs, patients who are dependent for at least one IADL are assumed to have IADL dependence. Frailty will be assessed using the Korean version of the fatigue, resistance, ambulation, illnesses, and loss of weight (K-FRAIL) questionnaire, with a score Ōēź 3 defined as having frailty [21]. In addition, gait speed and hand grip strength will also be measured to evaluate frailty. Gait speed will be measured according to the gait time of a 4-m course including 1-m of accelerated and decelerated course. The handgrip strength is to be measured two times in both arms using a TKK-5401 GRIPD (Takei, Niigata, Japan). Cognitive function will be assessed using the Korean version of the Montreal Cognitive Assessment (MoCA-K; range, 0 to 30; with lower numbers indicating worse function) [22]. Evaluation of health-related QOL will be performed using the Korean version of the EQ-5D [23].
Blood and urine tests, including complete blood cell count, liver enzymes, kidney function, and spot urine microalbumin, will be performed at an accredited institution. For patients with diabetes or suspected of having diabetes, HbA1c will also be included.
Antihypertensive medications will be prescribed according to the investigatorŌĆÖs judgement. The protocol recommends that physicians choose the following medications as first-line therapy for patients without comorbidities: calcium channel blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and diuretics. For patients with other compelling indications, medication will be prescribed according to the 2018 KSH guidelines [16].
In drug-na├»ve patients with hypertension, monotherapy will be started at the randomization visit. Two different classes of antihypertensive medications can be added simultaneously according to the investigatorŌĆÖs judgement in patients with an SBP Ōēź 160 mmHg and aged < 75 years. In patients who were already taking antihypertensive medications, another antihypertensive medication will be added if their SBP is higher than the target. After one month, patients with an SBP within the target range (SBP Ōēż 130 mmHg in the intensive group and Ōēż 140 mmHg in the standard group) will maintain their previous medication therapy. In contrast, patients who do not achieve the target SBP in each group will be treated with a second antihypertensive medication and followed up every month until SBP is reduced below the target. Once patients reach the SBP goal, they can visit the hospital on a regular schedule. For patients with an SBP > 160 mmHg, two types of antihypertensive medications can be added at the same time, according to the investigatorŌĆÖs judgement. The combination regimen will follow the recommendations included in the KSH guidelines [16]. In the standard treatment group, antihypertensive medication will be down-titrated if the clinic SBP is < 130 mmHg at regular visits or < 135 mmHg at two consecutive visits. During the study period, active dose titration according to the target BP will be recommended to increase the BP difference between the two groups, and the mean BPs of each treatment group will be periodically checked and communicated to all researchers.
The primary outcome of this study is a composite of ACS (myocardial infarction [MI] and unstable angina), stroke, heart failure, or death from CV causes. Strokes, which include ischemic and hemorrhagic strokes, and transient ischemic attacks are defined as a case of sudden local neurological disorder or consciousness disturbance due to a cerebrovascular disorder (occlusion of cerebral blood vessels, cerebral ischemia, cerebral infarction, rupture of cerebral blood vessels, or cerebral hemorrhage) that persists for more than 24 hours. Heart failure is defined as a hospitalization or emergency room visit requiring intravenous diuretic therapy. All left ventricular ejection fraction ranges will be included.
The secondary outcomes are prespecified and mainly include the following: (1) the occurrence of each component of the primary outcome and all-cause death; (2) hospitalization caused by CVD, such as ACS, heart failure, and stroke; (3) QOL assessed using the EQ-5D; (4) decline in cognitive function assessed using the MoCA-K; and (5) worsening frailty assessed using the frailty questionnaire, gait speed, and grip strength. The safety outcomes are also prespecified as follows: (1) development of renal dysfunction, defined as a Ōēź 50% reduction in the eGFR in a patient with stage 1 or 2 chronic kidney disease (CKD) (eGFR Ōēź 60 mL/min/1.73 m2), a Ōēź 30% reduction in the eGFR in a patient with stage 3 CKD (eGFR 30 to 59 mL/min/1.73 m2), or maintenance dialysis for more than 3 months or a history of kidney transplantation in a patient with stage 4 CKD (eGFR < 30 mL/min/1.73 m2); (2) decline in renal function during the study period, defined as an increase in serum creatinine Ōēź 0.3 mg/dL; (3) occurrence of orthostatic hypotension, defined as a decline of SBP Ōēź 20 mmHg or DBP Ōēź 10 mmHg within 3 minutes of standing after 5 minutes of lying in the supine position; (4) hypotension defined as an SBP < 90 mmHg; (5) dizziness; (6) syncope; (7) electrolyte imbalance; and (8) a fall.
To calculate the sample size, we used previous studies performed in Asian countries [7,8,13,24ŌĆō26]. In these studies, the incidence of major CV events was between 0.6% and 3.6%. However, given the difference in ethnicity and definition of CV events, this incidence could not be applied directly to our study. Therefore, we used data from the National Health Information Database (NHID) of the National Health Insurance Service (NHIS). According to the NHID from 2008 to 2013, the incidence of major CV events in elderly patients with hypertension aged Ōēź 65 years was 19.8% for 3 years in Korea. We assumed the difference in the primary outcome incidence between the two treatment groups would be more than 4% during the study period, which means the incidence of the primary outcome is expected to be 15.8% of the event rate per year in the intensive group and 19.8% in the standard group. We assumed a 2-year recruitment period, a maximum follow-up of 5 years, and a loss to follow-up rate of 2% per year. We have thus estimated a total of 1,588 subjects per group (3,176 in total) would be required to detect differences, with a two-sided ╬▒ level of 5% and 80% power. We thus plan to recruit a total of 3,176 patients (1,588 in each group).
Measured variables will be expressed as the mean ┬▒ standard deviation (SD) for continuous data and percentages for categorical data. The baseline characteristics of the intensive and standard treatment groups will be compared using the StudentŌĆÖs t test for continuous data and PearsonŌĆÖs chi-square test for categorical data. When the target recruitment period is over and the target participants are fully collected, Kaplan-Meier curves will be used to evaluate the time-to-event outcomes (ACS, stroke, heart failure, or death from CV cause). The risk of developing each event in the intensive treatment group compared to the standard treatment group will be estimated using the Cox proportional hazard model with a hazard ratio (HR) and 95% confidence interval (CI). To further evaluate the demographic and clinical characteristics, stratification and sensitivity analyses are also planned. Variables measured multiple times during follow-up will be analyzed using repeated-measures regression analyses.
Analysis of the primary and secondary outcomes will be conducted in three populations: (1) the intention-to-treatment (ITT) population, all patients who are assigned to the two treatment arms will be included; (2) the as-treated (AT) population, according to treatment type regardless of randomization; and (3) the per-protocol (PP) population, restriction of the patients who complete the trial. The outcomes will be analyzed in the ITT population as the primary analysis and in the AT and PP populations as secondary and repeated analyses.
An independent data monitoring committee (IDMC) and a safety monitoring committee have been organized to evaluate the progress of the trial in relation to the efficacy and safety of interventions during the study period. A primary interim analysis will be performed after 50% of the subjects are registered and a 1-year follow-up is completed.
All statistical analyses will use a significance level of 0.05 and will be performed using the SAS software program version 9.4 (SAS Institute Inc., Cary, NC, USA).
Patients have been enrolled in the HOWOLD-BP study since January 15, 2019. As of December 31, 2021, 1,655 patients have been registered, including 824 patients in the intensive treatment group and 832 patients in the standard treatment group. Participants consist of 783 men and 872 women. Thus far, 83 participants have completed the study, and 1,572 patients are in the follow-up period.
Treatment of hypertension is essential to reduce complications and mortality associated with hypertension. In this context, establishing an optimal SBP target is needed to provide appropriate treatment, especially in elderly patients, considering their specific characteristics, such as high pulse pressure, multiple comorbidities, frailty, and limited capacity for self-care. The HOWOLD-BP trial is a multicenter, randomized controlled trial that will include 3,176 patients with hypertension aged Ōēź 65 years from 11 clinical centers in Korea and aims to investigate whether intensive treatment (SBP Ōēż 130 mmHg) will provide more benefits in lowering CV events than standard treatment (SBP Ōēż 140 mmHg).
Previous studies have demonstrated that treatment of hypertension could reduce CV complications in the elderly [6,27ŌĆō30]. The Hypertension in the Very Elderly Trial (HYVET) randomly assigned 3,845 patients aged Ōēź 80 years (mean age 83.6 years) with an SBP Ōēź 160 mmHg (mean BP 173.0/90.8 mmHg) to receive either the indapamide (┬▒ perindopril) or matching placebo treatment. The target BP of the active treatment group was 150/80 mmHg and the SBP and DBP at 2 years were 143.5 and 77.9 mmHg in the active treatment group and 158.5 and 84.0 mmHg in the placebo group, respectively. Active treatment was associated with a 30% reduction in the rate of fatal and nonfatal stroke (primary outcome), a 21% reduction in the rate of death from any cause, and a 23% reduction in the rate of death from CV causes. Furthermore, fewer serious adverse events were reported in the active treatment group than in the placebo group [6]. Moreover, there was no evidence that frailty modifies the positive impact of antihypertensive treatment in this very elderly population, although this population mainly consisted of robust subjects [27].
In the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension) study, 1,627 patients aged 70 to 84 years (mean age 75.7 years; mean BP 195/102 mmHg) were randomized into either the active antihypertensive therapy group (three beta-blockers and one diuretic) or the placebo group. At 4 years, the mean SBP and DBP were 166 and 85 mmHg in the active treatment group versus 193 and 95 mmHg in the placebo group, respectively. The primary outcomes (stroke, MI, and other CV deaths) were significantly reduced in the active treatment group (relative risk, 0.60; 95% CI, 0.43 to 0.85) [28]. The other two trials, the Systolic Hypertension in the Elderly Program study (SHEP) [29] and the Systolic Hypertension in Europe (Syst-Eur) study [30], both of which included patients aged Ōēź 60 years with isolated systolic hypertension, also showed significant reductions in the incidence of total stroke and major CV events in the active treatment group. In these studies, consistent clinical benefits were seen with antihypertensive treatment in elderly patients similar to those seen in younger hypertensive patients, especially for those with an SBP Ōēź 160 mmHg at the time of study participation, even considering baseline BP level, individual CV risk, and comorbidities. However, any additional benefit associated with lowering the SBP below 140 mmHg in elderly patients with hypertension is still controversial.
Several large-scale clinical trials have evaluated the benefits of intensive BP-lowering therapy in elderly populations (Table 3). The Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS) trial enrolled 4,418 elderly hypertensive patients aged 65 to 85 years (mean age 73.6 years) with an SBP > 160 mmHg and compared the effects of 2 years of strict antihypertensive treatment (target SBP < 140 mmHg) with those of mild antihypertensive treatment (target SBP between 140 and 160 mmHg) [7]. The mean SBP was 135.9 mmHg in the strict treatment group and 145.6 mmHg in the mild treatment group at the end of the study. There was no significant difference between the two treatment strategies regarding the primary outcome, which was the combined incidence of cerebrovascular disease, cardiac and vascular disease, and acute or chronic renal failure. Moreover, according to the subgroup analysis of patients aged Ōēź 75 years, the incidence of the primary outcome tended to be higher in the strict treatment group than in the mild treatment group. Similarly, in the Valsartan in Elderly Isolated Systolic Hypertension Study (VALISH), which enrolled 3,260 patients with isolated systolic hypertension aged between 70 and 84 years (mean age, 76.1 years), strict BP control (SBP < 140 mmHg) was not superior to moderate BP control (SBP 140 to 160 mmHg) for reducing CV events [8]. The participants of these two trials showed a relatively low prevalence of CVD and diabetes, resulting in a low incidence of the primary outcome.
However, in a small randomized trial conducted in China [25], intensive antihypertensive treatment with a target BP Ōēż 140/90 mmHg resulted in a significant decrease in stroke and CV events compared to the standard treatment with a target BP Ōēż 150/90 mmHg. A total of 724 patients aged 70 years or older (mean age, 76.6 years) were enrolled, and the average SBP was 135.7 mmHg in the intensive treatment group and 149.7 mmHg in the standard treatment group over a mean follow-up of 4 years. Intensive treatment reduced the primary outcomes (a composite of stroke, acute MI, and other CV deaths) by 40.6%, total strokes by 42.0%, and heart failure-related deaths by 62.7%. The total and CV-related mortality rates also decreased by 41.7% and 50.3%, respectively, in the intensive treatment group.
A following larger subgroup analysis of elderly participants (aged Ōēź 75 years) who enrolled in the Systolic Blood Pressure Intervention Trial (SPRINT) also showed a significant benefit of intensive BP control (SBP target < 120 mmHg) compared to standard BP control (SBP target < 140 mmHg) [10]. Among the 9,361 participants in the original SPRINT trial, 2,636 were aged Ōēź 75 years (mean age, 79.9 years). The mean SBP was 123.4 mmHg in the intensive treatment group and 134.8 mmHg in the standard treatment group over a median follow-up of 3.14 years. The intensive treatment group showed a 34% lower rate of the primary outcome (a composite of nonfatal MI, ACS, nonfatal stroke, nonfatal acute decompensated heart failure, and death from CV causes) and a 33% lower rate of all-cause mortality. Surprisingly, the overall rate of serious adverse events was not different between the treatment groups, although the absolute rates were slightly higher in the intensive treatment group. Although there were higher rates of clinical events with increasing frailty in the exploratory subgroup analysis, frailty status did not modify the effect of intensive BP treatment on CV-related benefits. Even though the strength of the elderly participants aged Ōēź 75 years was a prespecified subgroup of the SPRINT trial and all participants enrolled in this study were at high risk for CV events, the generalizability of the results is somewhat limited because subjects with diabetes or prevalent stroke were excluded from the original SPRINT study [31].
The most recently published study, the Strategy of Blood Pressure Intervention in the Elderly Hypertensive Patients (STEP), also showed a significant benefit of intensive BP-lowering treatment (target SBP between 110 and 130 mmHg) compared to standard treatment (target SBP between 130 and 150 mmHg). A total of 8,511 Chinese patients with hypertension aged between 60 and 80 years (mean age, 66.2 years) were enrolled, and the mean SBP was 126.7 mmHg in the intensive treatment group and 135.9 mmHg in the standard treatment group over a median follow-up of 3.34 years [11]. The incidence of the primary outcome (a composite of stroke, ACS, acute decompensated heart failure, coronary revascularization, atrial fibrillation, and death from CV causes) was significantly lower in the intensive treatment group than in the standard treatment group (3.5% vs. 4.6%: HR, 0.74; p = 0.007). Most of the individual components of the primary endpoints also favored intensive treatment. The STEP trial is the largest randomized controlled trial to test whether intensive BP-lowering treatment has a beneficial effect on CV events, particularly in the elderly population. However, patients with prevalent atrial fibrillation or stroke were excluded from the STEP study. Although there was no significant interaction between the treatment group and age in the prespecified subgroup analysis according to age (< 70 years vs. Ōēź 70 years), the enrolled patients were relatively younger than those in the other trials for elderly patients with hypertension, with only 24% of the participants aged Ōēź 70 years. Though frailty status evaluated using the Fried phenotype and cognitive dysfunction using the Mini-Mental State Examination (MMSE) were measured according to the study protocol, the results of these assessments were not presented in the main results [11,32].
The inconsistent results regarding the beneficial effects of intensive BP treatment in the elderly among the previous studies might be attributable to differences in the achieved reduction in the SBP and features of the study population, such as underlying CV risk, comorbidities, and frailty. Moreover, the positive results of the recently published SPRINT-SENIOR [10] and STEP [11] studies cannot be used to represent patients with a history of stroke. Except for the SPRINT-SENIOR study, none of the previously mentioned trials have evaluated their results according to frailty status. Thus, the optimal target BP for elderly patients has been debated in recent years. Several specific factors should be considered when treating elderly patients [17]. Elderly patients often have multiple co-existing issues and concerns, including frailty, multiple comorbidities, polypharmacy, cognitive impairment, depression, disability, dizziness, syncope, and falls. Sometimes, these factors can complicate the treatment of hypertension. Thus, elderly patients with hypertension are heterogeneous, and chronological age is not necessarily synonymous with biological age.
Several features of the HOWOLD-BP design are worthy of note. First, the inclusion criteria allow most elderly patients aged Ōēź 65 years with hypertension to participate, as there is no upper age limit and only those who have ADL dependence will be excluded for ethical reasons. This open age criterion could, therefore, allow for an assessment of older members of the elderly population compared with that of the JATOS, VALISH, and STEP trials. In this study, there are also no specific exclusion criteria, such as history of atrial fibrillation, stroke, or diabetes, except for conditions that could influence the BP and treatment regimen. The choice to use a target SBP Ōēż 140 mmHg for the standard treatment group was based on current hypertension guidelines [15,16], and the target SBP Ōēż 130 mmHg used for the intensive treatment group was based on the SBP achieved in previous trials, which was the lowest in the SPRINT-SENIOR study (123.4 mmHg), and safety concerns with reducing the BP too low in the elderly population. The recommended treatment protocol according to the assigned target SBP in this study is based on the KSH guidelines [16], but the attending physician will decide the choice of antihypertensive drugs and titration protocol. It is thought that giving the attending physician free decision-making capacity will better reflect real-world practice. Additionally, every participant will be requested to record their home BP profile using the same authorized device, and the home BP data will be analyzed in conjunction with their corresponding clinic BP data. Considering the high prevalence of white-coat hypertension in the elderly population, obtaining home BP data is important for verifying the beneficial effects of intensive treatment. Additionally, assessing frailty status based on the questionnaire, gait speed, and handgrip strength in the HOWOLD-BP study is more comprehensive compared with previous studies. Cognitive function and health-related QOL will also be assessed.
However, we have encountered some problems in conducting this clinical study. Above all, the rate of patient enrollment has been lower than expected. To address this problem, we are constantly pushing for the involved institutions to enroll more patients. In addition, we plan to extend the follow-up period to 5 years to obtain sufficient events to assess the outcomes. Interim analyses will soon be conducted.
In conclusion, the HOWOLD-BP trial will be the first study performed in Korea to evaluate the beneficial effect of intensive treatment (SBP Ōēż 130 mmHg) compared with standard treatment (SBP Ōēż 140 mmHg) on CV outcomes in elderly patients with hypertension. The results of the HOWOLD-BP trial will provide new evidence of the optimal target BP for improving CV outcomes in elderly patients with hypertension, particularly in Asian populations.
1. The optimal target for lowering systolic blood pressure (SBP) to reduce cardiovascular disease (CVD) in elderly Korean patients with hypertension is unclear.
2. The HOW to Optimize eLDerly systolic Blood Pressure (HOWOLD-BP) trial is a large-scale, randomized controlled trial meant to compare CVD incidence in elderly Korean patients with hypertension based on two target SBP levels (Ōēż 130 and Ōēż 140 mmHg).
3. The results of the HOWOLD-BP trial will provide new evidence of the optimal target SBP for improving cardiovascular outcomes in elderly Korean patients with hypertension.
Acknowledgments
This work was supported by the research grant of Korea National Institute of Health (grant number: 2021-ER0901, 2021ŌĆō2023).
Figure┬Ā1
Diagram of the HOW to Optimize eLDerly systolic Blood Pressure (HOWOLD-BP) trial. SBP, systolic blood pressure; CV, cardiovascular.
Table┬Ā1
Measures and frequency during the follow-up procedure in HOWOLD-BP trial
Table┬Ā2
Inclusion and exclusion criteria of the HOWOLD-BP trial
Table┬Ā3
Characteristics of clinical trials for intensive versus standard BP lowering in the elderly
| JATOS [7] | VALISH [8] | Wei et al. [25] | SPRINT-SENIOR [10] | STEP [11] | |
|---|---|---|---|---|---|
| Published year | 2008 | 2010 | 2013 | 2016 | 2021 |
| Location | Japan | Japan | China | North America | China |
| Participants | 4,418 | 3,079 | 724 | 2,636 | 8,511 |
| Age criterion, yr | 65ŌĆō85 | 70 Ōēż age < 85 | Older than 70 | 75 or older | 60ŌĆō80 |
| Target BP, mmHg | SBP < 140 vs. 140 Ōēż SBP < 160 | SBP < 140 vs. 140 Ōēż SBP < 150 | BP Ōēż 140/90 vs. BP Ōēż 150/90 | SBP < 120 vs. SBP < 140 | 110 Ōēż SBP < 130 vs. 130 Ōēż SBP < 150 |
| Follow-up duration, yr | Median 2 | Median 3.07 | Mean 4 | Median 3.14 | Median 3.26 |
| Achieved SBP, mmHg | 135.9 vs. 145.6 | 136.6 vs. 142.0 | 135.7 vs. 149.7 | 123.4 vs. 134.8 | 126.7 vs. 135.9 |
| Primary endpoint | A composite of cerebrovascular disease (cerebral hemorrhage, cerebral infarction, transient ischemic attack, and subarachnoid hemorrhage), cardiac and vascular disease (myocardial infarction, angina requiring hospitalization, heart failure, sudden death, dissecting aneurysms, and occlusive arterial disease), and acute or chronic renal failure | A composite of cardiovascular events including sudden death, fatal or nonfatal stroke, fatal or nonfatal myocardial infarction, heart failure death, other cardiovascular death, unplanned hospitalization for cardiovascular disease and renal disorder | A composite of fatal/nonfatal stroke, acute myocardial infarction, and other cardiovascular deaths (sudden death and heart failure death) | A composite of nonfatal myocardial infarction, acute coronary syndrome not resulting in a myocardial infarction, nonfatal stroke, nonfatal acute decompensated heart failure, and death from cardiovascular cause | A composite of stroke, acute coronary syndrome (acute myocardial infarction and hospitalization for unstable angina), acute decompensated heart failure, coronary revascularization, atrial fibrillation, or death from cardiovascular causes |
| History of cardiovascular disease | 7.4% | 13.3% | 6.6%a | 24.5% | 6.3% |
| History of diabetes | 11.8% | 13.0% | 23.3% | 0% | 19.1% |
| Specific exclusion | Atrial fibrillation | Chronic kidney disease | Diabetes, stroke | Stroke, atrial fibrillation |
BP, blood pressure; JATOS, Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients; VALISH, Valsartan in Elderly Isolated Systolic Hypertension Study; SPRINT-SENIOR, Systolic Blood Pressure Intervention Trial-SENIOR; STEP, Strategy of Blood Pressure Intervention in the Elderly Hypertensive Patients; SBP, systolic blood pressure.
REFERENCES
1. Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ, Comparative Risk Assessment Collaborating Group. Selected major risk factors and global and regional burden of disease. Lancet 2002;360:1347ŌĆō1360.


2. Kannel WB. Historic perspectives on the relative contributions of diastolic and systolic blood pressure elevation to cardiovascular risk profile. Am Heart J 1999;138(3 Pt 2):205ŌĆō210.


3. Ostchega Y, Fryar CD, Nwankwo T, Nguyen DT. Hypertension prevalence among adults aged 18 and over: United States, 2017ŌĆō2018. NCHS Data Brief 2020;364:1ŌĆō8.
4. Kim HC, Cho SM, Lee H, et al. Korea hypertension fact sheet 2020: analysis of nationwide population-based data. Clin Hypertens 2021;27:8.




5. Staessen JA, Wang JG, Thijs L. Cardiovascular protection and blood pressure reduction: a meta-analysis. Lancet 2001;358:1305ŌĆō1315.


6. Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008;358:1887ŌĆō1898.


7. JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008;31:2115ŌĆō2127.


8. Ogihara T, Saruta T, Rakugi H, et al. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension 2010;56:196ŌĆō202.


9. SPRINT Research Group; Wright JT Jr, et al.; Williamson JD. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373:2103ŌĆō2116.



10. Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged Ōēź75 years: a randomized clinical trial. JAMA 2016;315:2673ŌĆō2682.



11. Zhang W, Zhang S, Deng Y, et al. Trial of intensive blood-pressure control in older patients with hypertension. N Engl J Med 2021;385:1268ŌĆō1279.


12. Bavishi C, Bangalore S, Messerli FH. Outcomes of intensive blood pressure lowering in older hypertensive patients. J Am Coll Cardiol 2017;69:486ŌĆō493.


13. Liu L, Zhang Y, Liu G, et al. The Felodipine Event Reduction (FEVER) Study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. J Hypertens 2005;23:2157ŌĆō2172.


14. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 2018;138:e426ŌĆōe483.

15. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021ŌĆō3104.

16. Lee HY, Shin J, Kim GH, et al. 2018 Korean Society of Hypertension guidelines for the management of hypertension: part II-diagnosis and treatment of hypertension. Clin Hypertens 2019;25:20.




17. Lee JH, Kim KI, Cho MC. Current status and therapeutic considerations of hypertension in the elderly. Korean J Intern Med 2019;34:687ŌĆō695.




18. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 2011;21:69ŌĆō72.



19. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179ŌĆō186.


20. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373ŌĆō383.


21. Jung HW, Yoo HJ, Park SY, et al. The Korean version of the FRAIL scale: clinical feasibility and validity of assessing the frailty status of Korean elderly. Korean J Intern Med 2016;31:594ŌĆō600.




22. Lee JY, Lee DW, Cho SJ, et al. Brief screening for mild cognitive impairment in elderly outpatient clinic: validation of the Korean version of the Montreal Cognitive Assessment. J Geriatr Psychiatry Neurol 2008;21:104ŌĆō110.



23. Kim MH, Cho YS, Uhm WS, Kim S, Bae SC. Cross-cultural adaptation and validation of the Korean version of the EQ-5D in patients with rheumatic diseases. Qual Life Res 2005;14:1401ŌĆō1406.



24. Ogihara T, Saruta T, Matsuoka H, et al. valsartan in elderly isolated systolic hypertension (VALISH) study: rationale and design. Hypertens Res 2004;27:657ŌĆō661.


25. Wei Y, Jin Z, Shen G, et al. Effects of intensive antihypertensive treatment on Chinese hypertensive patients older than 70 years. J Clin Hypertens (Greenwich) 2013;15:420ŌĆō427.




26. Zhang Y, Zhang X, Liu L, Zanchetti A, FEVER Study Group. Is a systolic blood pressure target <140 mmHg indicated in all hypertensives?: subgroup analyses of findings from the randomized FEVER trial. Eur Heart J 2011;32:1500ŌĆō1508.


27. Warwick J, Falaschetti E, Rockwood K, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the HYpertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med 2015;13:78.


28. Dahlof B, Lindholm LH, Hansson L, Schersten B, Ekbom T, Wester PO. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension). Lancet 1991;338:1281ŌĆō1285.


29. SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991;265:3255ŌĆō3264.


30. Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997;350:757ŌĆō764.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



